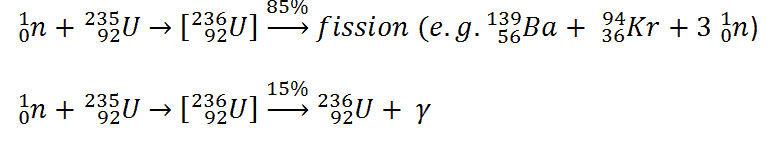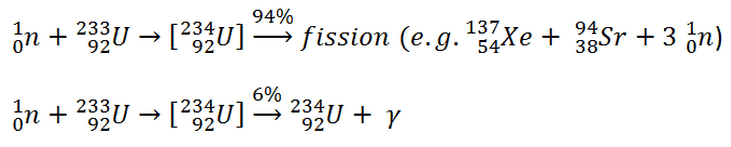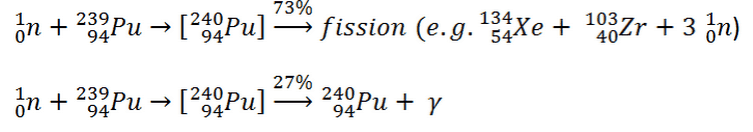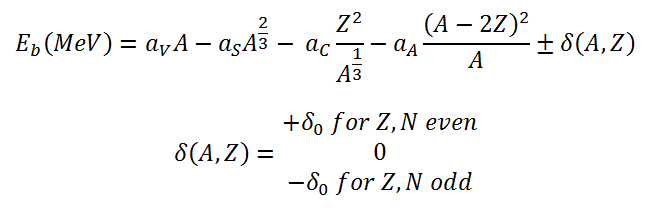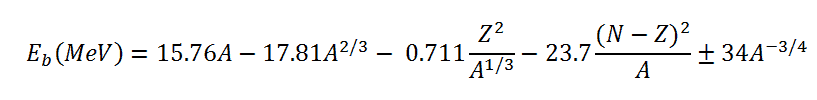Article Summary & FAQs
What is nuclear fission?
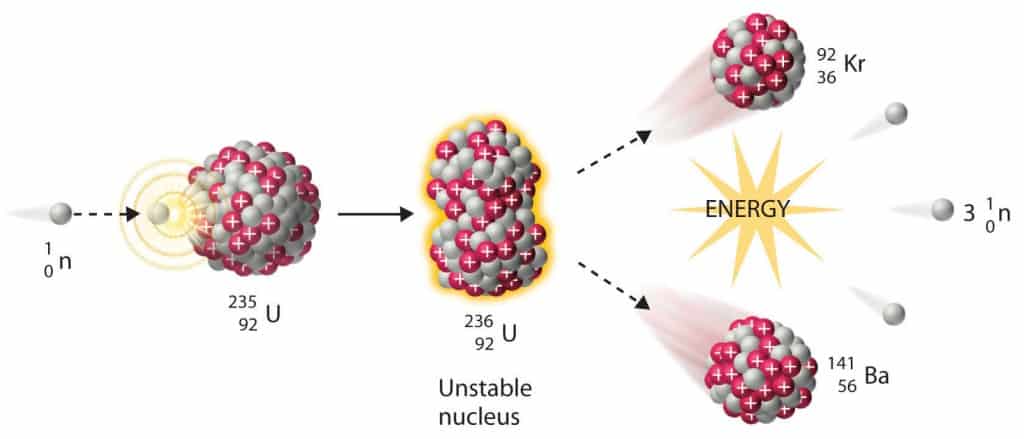
Nuclear fission is a nuclear reaction or a decay process in which the heavy nucleus splits into smaller parts (lighter nuclei). The fission process often produces free neutrons, photons (in the form of gamma rays) and releases a large amount of energy.
Key Facts
- The case of the nuclear decay process is called spontaneous fission, and it is a sporadic process. Neutron-induced nuclear fission is the process of the most significant practical importance in reactor physics.
- Some nuclei can undergo fission on their own spontaneously, but only specific nuclei, like uranium-235, uranium-233, and plutonium-239, can sustain a fission chain reaction.
- The fission process may produce 2, 3, or more free neutrons, and these neutrons can trigger further fission, and a chain reaction can occur.
- The total energy released in a reactor is about 210 MeV per 235U fission.
- About 3.1⋅1010 fissions per second are required to produce a power of 1 W.
- Since 1 gram of any fissile material contains about 2.5 x 1021 nuclei, the fissioning of 1 gram of fissile material yields about one megawatt-day (MWd) of heat energy.
- In 2011 nuclear power provided 10% of the world’s electricity. In 2007, the IAEA reported 439 nuclear power reactors operating globally, operating in 31 countries.
See also: Ternary Fission
Key Characteristics of Nuclear Fission
- Nuclear fission is the primary process of generating nuclear energy.
- Most of the energy (~85%) is released in the form of the kinetic energy of the split parts.
- Neutrons trigger nuclear fission.
- The fission process produces free neutrons (2 or 3).
- The chain reaction means if the reaction induces one or more reactions.
- The probability that fission will occur depends on incident neutron energy.
- Therefore the moderator is used to slow down neutrons (to increase the probability of fission)
- For reactors using light water as moderators, enriched uranium fuel is required.
- Control rods contain material that absorbs neutrons (boron, cadmium, …)
- Withdrawal of the rods increases the parameter one or more (multiplication factor), thus increase the power.
- Insertion of the rods decreases the parameter one or more (multiplication factor), thus decrease the power.
- The multiplication factor is also influenced by other parameters such as temperature, fuel burnup, and reactor poisoning.
- The fission takes place in the nuclear reactor core.
- The reactor core consists of nuclear fuel, control rods, moderator (coolant), and neutron reflector.
Nuclear Fission and Chain Reaction
Some nuclei can undergo fission on their own spontaneously, but only specific nuclei, like uranium-235, uranium-233, and plutonium-239, can sustain a fission chain reaction. This is because these nuclei release neutrons when they break apart, which can induce fission of other nuclei. Free neutrons released by each fission play a critical role as a trigger of the reaction.
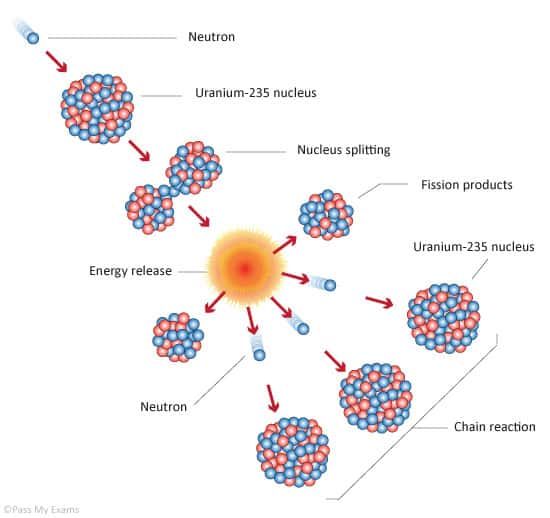
Chain reaction
A nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The “one or more” is the key parameter of reactor physics. The amount of reactions must be changed (using the control rods) to raise or lower the power so that the number of neutrons present (hence the power generation rate) is either reduced or increased.
Physics of Nuclear Fission
In general, the neutron-induced fission reaction is the reaction in which the incident neutron enters the heavy target nucleus (fissionable nucleus), forming a compound nucleus that is excited to such a high energy level (Eexcitation > Ecritical) that the nucleus splits into two large fission fragments. A large amount of energy is released in the form of radiation and fragment kinetic energy. Moreover, and what is crucial, the fission process may produce 2, 3, or more free neutrons, and these neutrons can trigger further fission, and a chain reaction can occur. To understand the process of fission, we must understand processes that occur inside the nucleus to be fissioned. At first, the nuclear binding energy must be defined.
Nuclear Binding Energy
At the nuclear level, the nuclear binding energy is required to disassemble (to overcome the strong nuclear force) a nucleus of an atom into its component parts (protons and neutrons). The protons and neutrons in an atomic nucleus are held together by the nuclear forces (strong force). The mass of a nucleus is always less than the sum of masses of the constituent protons and neutrons when separated. The difference is a measure of the nuclear binding energy (Eb) which holds the nucleus together. According to the Einstein relationship (E=m.c2) this binding energy is proportional to this mass difference, known as the mass defect.
Source: hyperphysics.phy-astr.gsu.edu
During the nuclear splitting or nuclear fusion, some of the mass of the nucleus gets converted into vast amounts of energy. Thus this mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. The nuclear binding energies are enormous, and they are on the order of a million times greater than the electron binding energies of atoms.
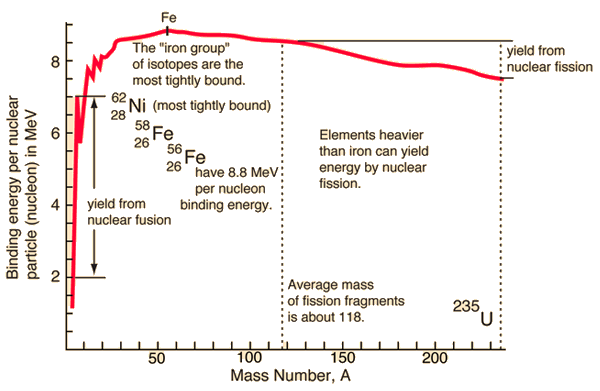
For a nucleus with A (mass number) nucleons, the binding energy per nucleon Eb/A can be calculated. This calculated fraction is shown in the chart as a function of the mass number A. As can be seen, for low mass numbers, Eb/A increases rapidly and reaches a maximum of 8.8 MeV at approximately A=60. The nuclei with the highest binding energies that are most tightly bound belong to the “iron group” of isotopes (56Fe, 58Fe, 62Ni). After that, the binding energy per nucleon decreases. A more stable configuration is obtained in the heavy nuclei (A>60) region when a heavy nucleus splits into two lighter nuclei. This is the origin of the fission process. It may seem that all the heavy nuclei may undergo fission or even spontaneous fission. In fact, for all nuclei with atomic number greater than about 60, fission occurs very rarely. For the fission process to take place, a sufficient amount of energy must be added to the nucleus, no matter how. The energetics and binding energies of a specific nucleus are well described by the Liquid Drop Model, which examines the global properties of nuclei.
Liquid Drop Model
 One of the first models that could describe the behavior of the nuclear binding energies. Therefore nuclear masses were the mass formula of von Weizsaecker (also called the semi-empirical mass formula – SEMF) published in 1935 by German physicist Carl Friedrich von Weizsäcker. This theory is based on the liquid drop model proposed by George Gamow.
One of the first models that could describe the behavior of the nuclear binding energies. Therefore nuclear masses were the mass formula of von Weizsaecker (also called the semi-empirical mass formula – SEMF) published in 1935 by German physicist Carl Friedrich von Weizsäcker. This theory is based on the liquid drop model proposed by George Gamow.
According to this model, the atomic nucleus behaves like the molecules in a drop of liquid. But in this nuclear scale, the fluid is made of nucleons (protons and neutrons), held together by the strong nuclear force. The liquid drop model of the nucleus considers that the nuclear forces on the nucleons on the surface are different from those on nucleons in the interior of the nucleus. Other attracting nucleons completely surround the interior nucleons. Here is the analogy with the forces that form a drop of liquid.
In the ground state, the nucleus is spherical. If sufficient kinetic or binding energy is added, this spherical nucleus may be distorted into a dumbbell shape and then maybe split into two fragments. Since these fragments are more stable, the splitting of such heavy nuclei must be accompanied by energy release. This model does not explain all the properties of the atomic nucleus but does explain the predicted nuclear binding energies.
The nuclear binding energy, as a function of the mass number A and the number of
protons Z, is based on the liquid drop model. Its formula can be written as:
This formula is called the Weizsaecker Formula (or the semi-empirical mass formula). The physical meaning of this equation can be discussed term by term.
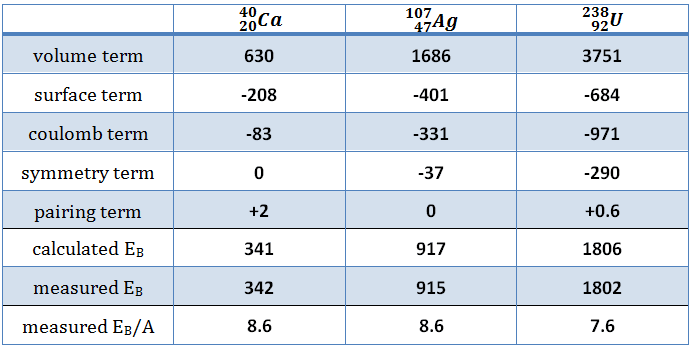
With the aid of the Weizsaecker formula, the binding energy can be calculated very well for nearly all isotopes. This formula provides a good fit for heavier nuclei. For light nuclei, especially for 4He, it provides a poor fit. The main reason is the formula does not consider the internal shell structure of the nucleus.
The coefficients aV, aS, aC, aA, and aP must be known to calculate the binding energy. The coefficients have units of megaelectronvolts (MeV) and are calculated by fitting to experimentally measured masses of nuclei. They usually vary depending on the fitting methodology. According to ROHLF, J. W., Modern Physics from α to Z0, Wiley, 1994., the coefficients in the equation are following:
Using the Weizsaecker formula, also the mass of an atomic nucleus can be derived and is given by:
m = Z.mp + N.mn – Eb/c2
where mp and mn are the rest mass of a proton and a neutron, respectively, and Eb is the nuclear binding energy of the nucleus.
From the nuclear binding energy curve and from the table, it can be seen that, in the case of splitting a 235U nucleus into two parts, the binding energy of the fragments (A ≈ 120) together is more significant than that of the original 235U nucleus.
According to the Weizsaecker formula, the total energy released for such a reaction will be approximately 235 x (8.5 – 7.6) ≈ 200 MeV.
See also: Liquid Drop Model
Critical Energy – Threshold Energy for Fission
In principle, any nucleus, if brought into a sufficiently high excited state, can be split. For fission to occur, the excitation energy must be above a particular value for a certain nuclide. The minimum excitation energy required for fission to occur is known as the critical energy (Ecrit) or threshold energy.
The critical energy depends on the nuclear structure and is significant for light nuclei with Z < 90. For heavier nuclei with Z > 90, the critical energy is about 4 to 6 MeV for A-even nuclei and generally is much lower for A-odd nuclei. It must be noted, some heavy nuclei (e.g.,, 240Pu or 252Cf) exhibit fission even in the ground state (without externally added excitation energy). This phenomenon is known as spontaneous fission. This process occurs without adding the critical energy by the quantum-mechanical process of quantum tunneling through the Coulomb barrier (similarly to alpha particles in the alpha decay). Spontaneous fission ensures sufficient neutron flux on source range detectors when the reactor is subcritical in long-term shutdown.
See also: Critical Energy – Threshold Energy for Fission.
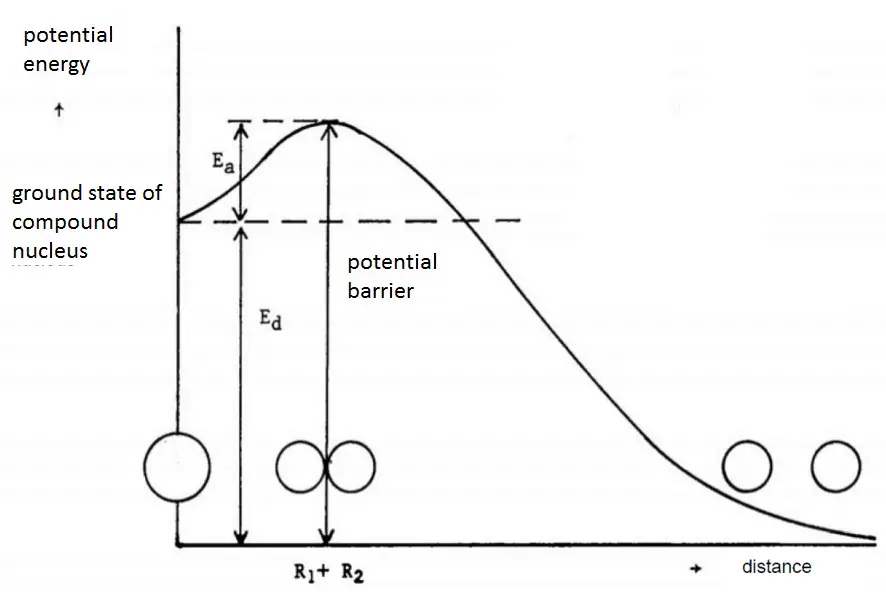
The minimum excitation energy required for fission to occur is known as the critical energy (Ecrit) or threshold energy.
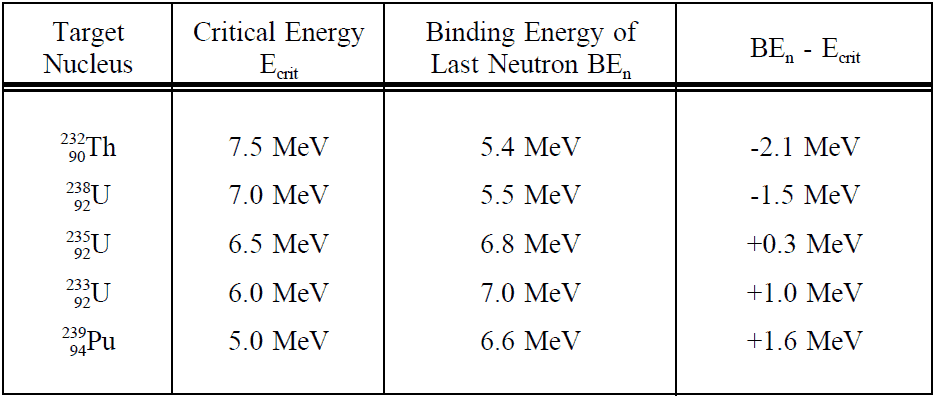
This table shows critical energies compared to binding energies of the last neutron of a number of nuclei.
Energy Release from Fission
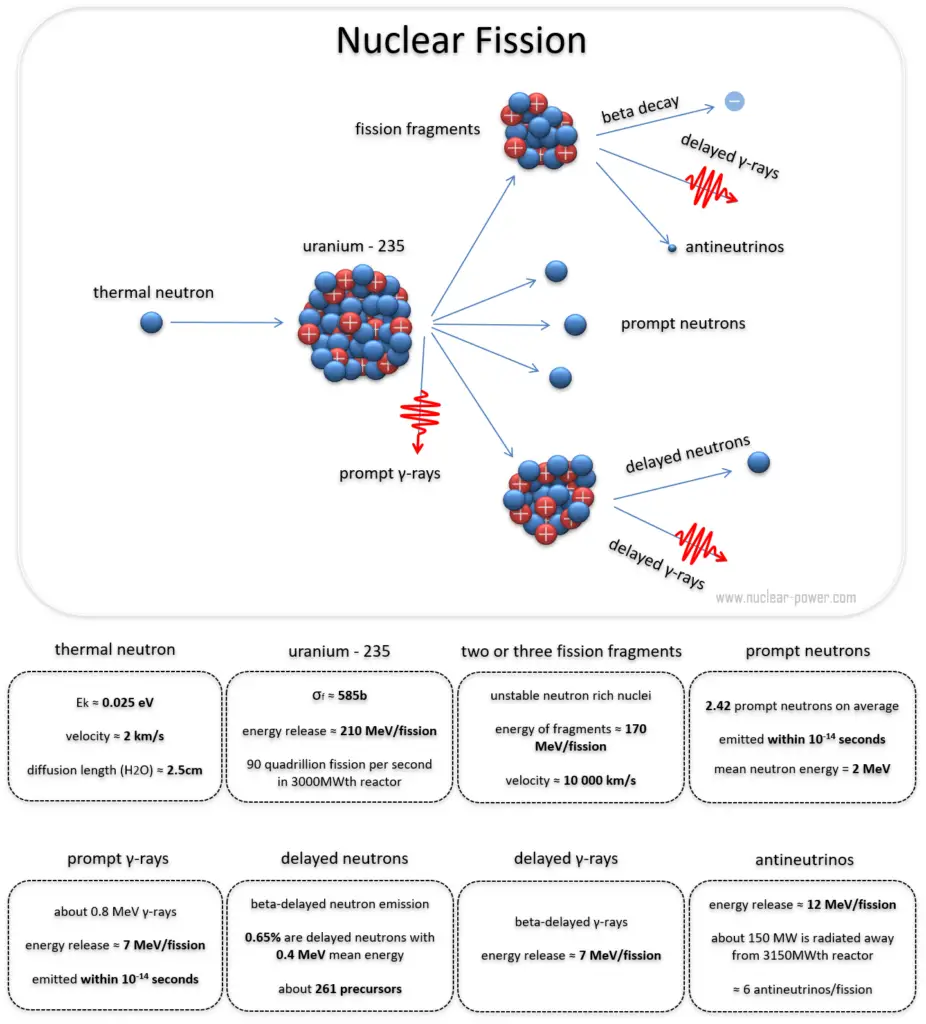
In general, nuclear fission results in the release of enormous quantities of energy. The amount of energy depends strongly on the nucleus fissioned and depends strongly on an incident neutron’s kinetic energy. To calculate the reactor’s power, it is necessary to accurately identify the individual components of this energy. At first, it is important to distinguish between the total energy released and the energy that can be recovered in a reactor.
The total energy released in fission can be calculated from binding energies of the initial target nucleus to be fissioned and binding energies of fission products. But not all the total energy can be recovered in a reactor. For example, about 10 MeV is released in the form of neutrinos (in fact antineutrinos). Since the neutrinos are weakly interacting (with extremely low cross-section of any interaction), they do not contribute to the energy that can be recovered in a reactor.
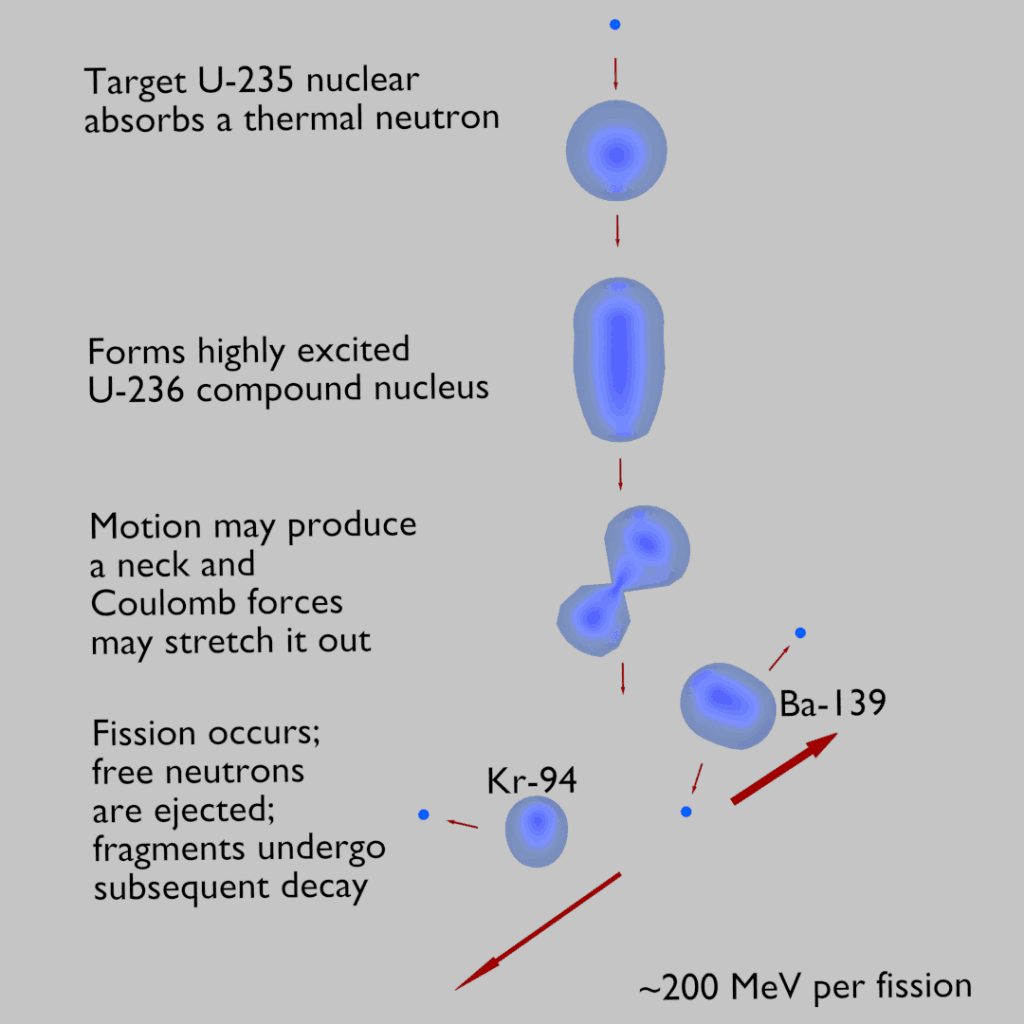
To understand this issue, we have first to investigate a typical fission reaction such as the one listed below.
Using this picture, we can identify and describe almost all the individual components of the total energy released during the fission reaction.
The existence of emission of antineutrinos and their extremely low cross-section for any interaction leads to a fascinating phenomenon.
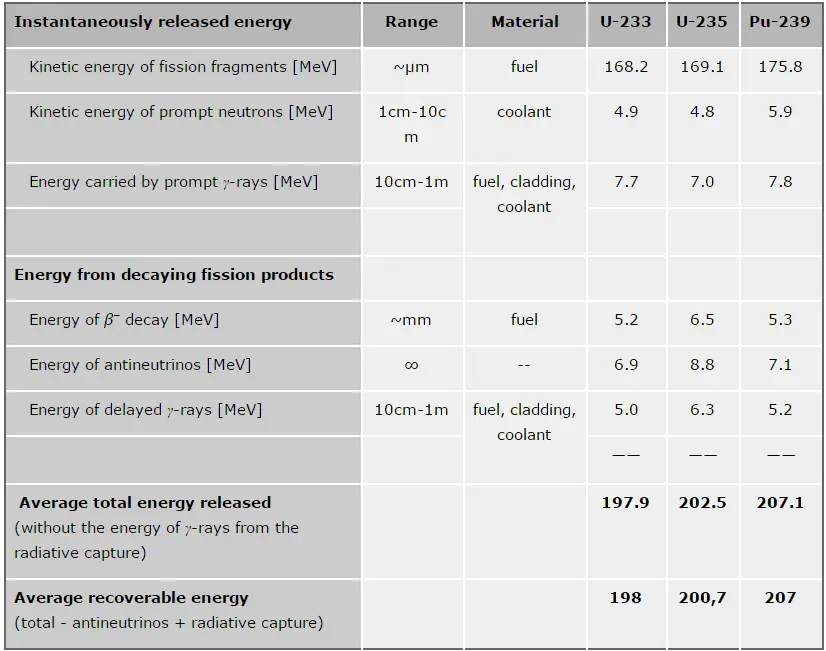
The total energy released in a reactor is about 210 MeV per 235U fission, distributed as shown in the table. The average recoverable energy per fission in a reactor is about 200 MeV (the total energy minus the energy of antineutrinos radiated away). This means that about 3.1⋅1010 fissions per second are required to produce a power of 1 W. Since 1 gram of any fissile material contains about 2.5 x 1021 nuclei, the fissioning of 1 gram of fissile material yields about one megawatt-day (MWd) of heat energy.
As can be seen from the description of the individual components of the total energy released during the fission reaction, there is a significant amount of energy generated outside the nuclear fuel (outside fuel rods). Especially the kinetic energy of prompt neutrons is primarily generated in the coolant (moderator). This phenomenon needs to be included in the nuclear calculations.
For LWR, it is generally accepted that about 2.5% of total energy is recovered in the moderator. This fraction of energy depends on the materials, their arrangement within the reactor, and thus on the reactor type.
Fission Fragments – Products of Nuclear Fission
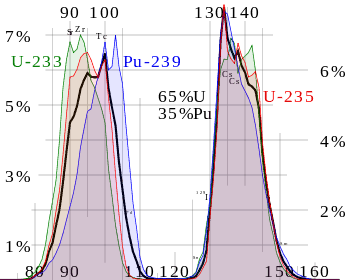
Nuclear fission fragments are the fragments left after a nucleus fissions. Typically, when the uranium 235 nucleus undergoes fission, the nucleus splits into two smaller nuclei, along with a few neutrons and release of energy in the form of heat (kinetic energy of these fission fragments) and gamma rays. The average of the fragment mass is about 118, but very few fragments near that average are found. It is much more probable to break up into unequal fragments, and the most probable fragment masses are around mass 95 (Krypton) and 137 (Barium).
Most of these fission fragments are highly unstable (radioactive) and undergo further radioactive decays to stabilize themselves. Fission fragments interact strongly with the surrounding atoms or molecules traveling at high speed, causing them to ionize.
See also: Interaction of Heavy Charged Particles with Matter
Prompt and Delayed Neutrons
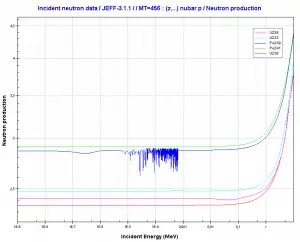
It is known the fission neutrons are of importance in any chain-reacting system. Neutrons trigger the nuclear fission of some nuclei (235U, 238U, or even 232Th). What is crucial the fission of such nuclei produces 2, 3, or more free neutrons.
But not all neutrons are released at the same time following fission. Even the nature of the creation of these neutrons is different. From this point of view, we usually divide the fission neutrons into two following groups:
- Prompt Neutrons. Prompt neutrons are emitted directly from fission, and they are emitted within a very short time of about 10-14 seconds.
- Delayed Neutrons. Delayed neutrons are emitted by neutron-rich fission fragments that are called delayed neutron precursors. These precursors usually undergo beta decay, but a small fraction of them are excited enough to undergo neutron emission. The neutron is produced via this type of decay, which happens orders of magnitude later than the emission of the prompt neutrons, which plays a crucial role in the control of the reactor.
See also: Prompt Neutrons
See also: Delayed Neutrons
See also: Reactor control with and without delayed neutrons – Interactive chart.
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Key Characteristics of Prompt Neutrons
- Prompt neutrons are emitted directly from fission, and they are emitted within a very short time of about 10-14 seconds.
- Most of the neutrons produced in fission are prompt neutrons – about 99.9%.
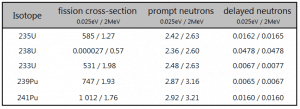
- For example, fission of 235U by thermal neutron yields 2.43 neutrons, of which 2.42 neutrons are prompt neutrons, and 0.01585 neutrons are the delayed neutrons.
- The production of prompt neutrons slightly increases with incident neutron energy.
- Almost all prompt fission neutrons have energies between 0.1 MeV and 10 MeV.
- The mean neutron energy is about 2 MeV. The most probable neutron energy is about 0.7 MeV.
- In reactor design, the prompt neutron lifetime (PNL) belongs to key neutron-physical characteristics of the reactor core.
- Its value depends primarily on the type of the moderator and the energy of the neutrons causing fission.
- In an infinite reactor (without escape), prompt neutron lifetime is the sum of the slowing downtime and the diffusion time.
- In LWRs, the PNL increases with the fuel burnup.
- The typical prompt neutron lifetime in thermal reactors is on the order of 10-4 seconds.
- The typical prompt neutron lifetime in fast reactors is on the order of 10-7 seconds.
Key Characteristics of Delayed Neutrons
- The presence of delayed neutrons is perhaps the most important aspect of the fission process from reactor control.
- Delayed neutrons are emitted by neutron-rich fission fragments that are called delayed neutron precursors.
- These precursors usually undergo beta decay, but a small fraction of them are excited enough to undergo neutron emission.
- The emission of neutrons happens orders of magnitude later compared to the emission of the prompt neutrons.
- About 240 n-emitters are known between 8He and 210Tl. About 75 of them are in the non-fission region.
- The grouping of precursors based on their half-lives is designed to simplify the kinetic calculations of the reactor.
- Therefore delayed neutrons are traditionally represented by six delayed neutron groups.
- Neutrons can also be produced in (γ, n) reactions (especially in reactors with heavy water moderator), and therefore, they are usually referred to as photoneutrons. Photoneutrons are usually treated no differently than regularly delayed neutrons in the kinetic calculations.
- The total yield of delayed neutrons per fission, vd, depends on:
- An isotope that is fissioned.
- The energy of a neutron induces fission.
- Variation among individual group yields is much more significant than variation among group periods.
- In reactor kinetic calculations, it is convenient to use relative units usually referred to as delayed neutron fraction (DNF).
- At the steady-state condition of criticality, with keff = 1, the delayed neutron fraction is equal to the precursor yield fraction β.
- In LWRs, the β decreases with fuel burnup. This is due to isotopic changes in the fuel.
- Delayed neutrons have initial energy between 0.3 and 0.9 MeV with an average energy of 0.4 MeV.
- Depending on the type of the reactor, and their spectrum, the delayed neutrons may be more (in thermal reactors) or less effective than prompt neutrons (in fast reactors). To include this effect into the reactor kinetic calculations, the effectively delayed neutron fraction – βeff must be defined.
- The effectively delayed neutron fraction is the product of the average delayed neutron fraction and the importance factor βeff = β . I.
- The weighted delayed generation time is given by τ = ∑iτi . βi / β = 13.05 s, therefore the weighted decay constant λ = 1 / τ ≈ 0.08 s-1.
- The mean generation time with delayed neutrons is about ~0.1 s, rather than ~10-5 as in section Prompt Neutron Lifetime, where the delayed neutrons were omitted.
- Their presence completely changes the dynamic time response of a reactor to some reactivity change, making it controllable by control systems such as the control rods.
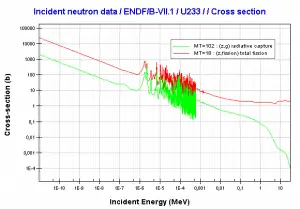
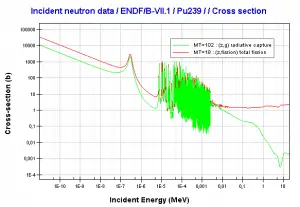
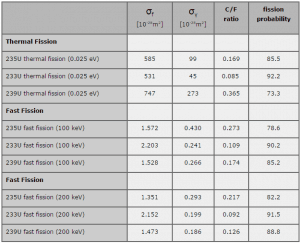
Capture-to-Fission Ratio
The probability that a neutron absorbed in a fissile nuclide causes a
fission is an important parameter of each fissile isotope. In terms of cross-sections, this probability is defined as:
σf / (σf + σγ) = 1 / (1 + σγ/σf) = 1 / (1 + α),
where α = σγ/σf is referred to as the capture-to-fission ratio. The capture-to-fission ratio may be used as an indicator of the “quality” of fissile isotopes. The lower C/F ratio simply means that an absorption reaction will result in fission rather than radiative capture. The ratio depends strongly on the incident neutron energy. In the fast neutron region, the C/F ratio decreases. It is determined by the steeper decrease in radiative capture cross-section (see chart).
For 235U and 233U, the thermal neutron capture-to-fission ratios are typically lower than fast neutrons (for mean energy of about 100 keV). It must be noted, the neutron flux of most fast reactors tends to peak around 200 keV, but the mean energy is between 100-200 keV depending on specific reactor design.
Further increase in neutron energy causes a decrease in the C/F ratio conversely. This is not the case with 239Pu. For 100 keV neutrons, the C/F ratio is lower than for thermal neutrons. A small capture-to-fission ratio is advantageous for the fissile isotopes (233U, 235U, and 239Pu) because neutrons captured onto them are lost.
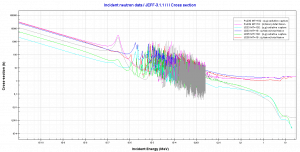
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Nuclear Fission Chain Reaction
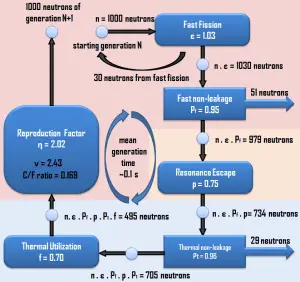 The chain reaction can take place only in the proper multiplication environment and only under proper conditions. Obviously, suppose one neutron causes two further fissions. In that case, the number of neutrons in the multiplication system will increase in time, and the reactor power (reaction rate) will also increase in time. Moreover, this multiplication environment (nuclear reactor) behaves like an exponential system, which means the power increase is not linear but exponential. The non-fission neutron absorption in the system (e.g.,, insert control rods) must increase to stabilize such a multiplication environment.
The chain reaction can take place only in the proper multiplication environment and only under proper conditions. Obviously, suppose one neutron causes two further fissions. In that case, the number of neutrons in the multiplication system will increase in time, and the reactor power (reaction rate) will also increase in time. Moreover, this multiplication environment (nuclear reactor) behaves like an exponential system, which means the power increase is not linear but exponential. The non-fission neutron absorption in the system (e.g.,, insert control rods) must increase to stabilize such a multiplication environment.
On the other hand, if one neutron causes less than one further fission, the number of neutrons in the multiplication system will decrease in time, and the reactor power (reaction rate) will also decrease in time. The non-fission neutron absorption in the system (e.g.,, to withdraw control rods) must be decreased to sustain the chain reaction.
There is always a competition for the fission neutrons in the multiplication environment. Some neutrons will cause further fission reaction, fuel materials or non-fuel materials will capture some, and some will leak out of the system.
The infinite and finite multiplication factors of a reactor must be defined to describe a multiplication system. The method of calculations of multiplication factors was developed in the early years of nuclear energy. It is only applicable to thermal reactors, where the bulk of fission reactions occurs at thermal energies. This method puts into context all the processes associated with the thermal reactors (e.g.,, neutron thermalization, neutron diffusion, or fast fission) because the most critical neutron-physical processes occur in energy regions that can be clearly separated from each other. In short, the calculation of the multiplication factor gives a good insight into the processes that occur in each thermal multiplying system.
The distinction between Fissionable, Fissile, and Fertile
In nuclear engineering, fissionable material (nuclide) is material that is capable of undergoing fission reaction after absorbing either thermal (slow or low energy) neutron or fast (high energy) neutron. Fissionable materials are a superset of fissile materials. Fissionable materials also include an isotope 238U that can be fissioned only with high energy (>1MeV) neutron. These materials are used to fuel thermal nuclear reactors because they can sustain a nuclear fission chain reaction.
Fissile materials undergo a fission reaction after absorption of the binding energy of the thermal neutron. They do not require additional kinetic energy for fission. If the neutron has higher kinetic energy, this energy will be transformed into the additional excitation energy of the compound nucleus. On the other hand, the binding energy released by the compound nucleus of (238U + n) after thermal neutron absorption is less than the critical energy, so the fission reaction cannot occur. The distinction is described in the following points.
- Fissile materials are a subset of fissionable materials.
- Fissionable material consists of isotopes that are capable of undergoing nuclear fission after capturing either fast neutron (high energy neutron – let say >1 MeV) or thermal neutron (low energy neutron – let say 0.025 eV). Typical fissionable materials: 238U, 240Pu, but also 235U, 233U, 239Pu, 241Pu
- Fissile material consists of fissionable isotopes capable of undergoing nuclear fission only after capturing a thermal neutron. Typical fissile materials: 235U, 233U, 239Pu, 241Pu. 238U is not a fissile isotope because a thermal neutron cannot fission 238U. 238U does not also meet alternative requirements to fissile materials. 238U cannot sustain a nuclear fission chain reaction because neutrons produced by the fission of 238U have lower energies than the original neutron (usually below the threshold energy of 1 MeV).
- Fertile material consists of isotopes that are not fissionable by thermal neutrons but can be converted into fissile isotopes (after neutron absorption and subsequent nuclear decay). Typical fertile materials: 238U, 232Th.
Fissile materials undergo a fission reaction after absorption of the binding energy of the thermal neutron. They do not require additional kinetic energy for fission. If the neutron has higher kinetic energy, this energy will be transformed into the additional excitation energy of the compound nucleus. On the other hand, the binding energy released by the compound nucleus of (238U + n) after absorption of the thermal neutron is less than the critical energy, so the fission reaction cannot occur. The distinction is described in the following points.
- Fissile materials are a subset of fissionable materials.
- Fissionable material consists of isotopes that are capable of undergoing nuclear fission after capturing either fast neutron (high energy neutron – let say >1 MeV) or thermal neutron (low energy neutron – let say 0.025 eV). Typical fissionable materials: 238U, 240Pu, but also 235U, 233U, 239Pu, 241Pu
- Fissile material consists of fissionable isotopes capable of undergoing nuclear fission only after capturing a thermal neutron. Typical fissile materials: 235U, 233U, 239Pu, 241Pu. 238U is not a fissile isotope because a thermal neutron cannot fission 238U. 238U does not also meet the alternative requirement to fissile materials. 238U cannot sustain a nuclear fission chain reaction because neutrons produced by the fission of 238U have lower energies than the original neutron (usually below the threshold energy of 1 MeV).
- Fertile material consists of isotopes that are not fissionable by thermal neutrons but can be converted into fissile isotopes (after neutron absorption and subsequent nuclear decay). Typical fertile materials: 238U, 232Th.
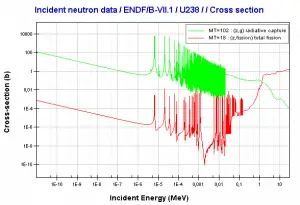
See also: Neutron cross-section.
Comparison of cross-sections
Source: JANIS (Java-based nuclear information software) http://www.oecd-nea.org/janis/
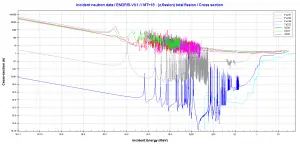
http://www.oecd-nea.org/janis/
Fissile / Fertile Material Cross-sections. Comparison of total fission cross-sections.
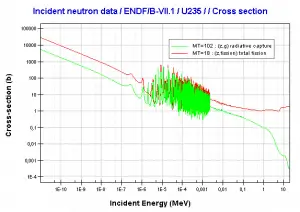 Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.
Uranium 235. Comparison of total fission cross-section and cross-section for radiative capture.
http://www.oecd-nea.org/janis/
Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.