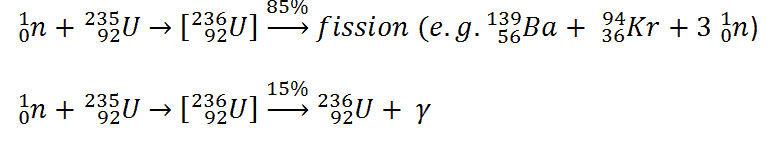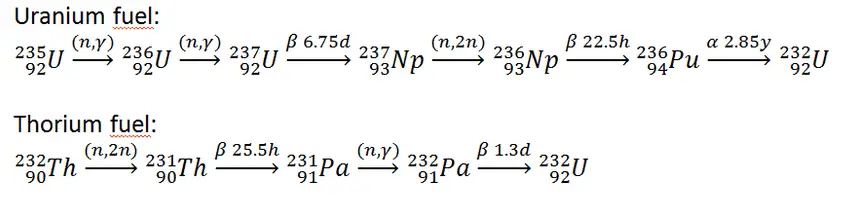What is Uranium
Uranium is a naturally occurring chemical element with atomic number 92, which means there are 92 protons and 92 electrons in the atomic structure. The chemical symbol for uranium is U. Uranium was discovered in 1789 by Martin Klaproth in the mineral called pitchblende (uraninite). He named the newly discovered element after the planet Uranus, which had been discovered eight years earlier. It was first isolated as a metal in 1841 by Eugene-Melchior Peligot. Henri Becquerel discovered uranium to be radioactive in 1896. He discovered that uranium minerals could expose a photographic plate through another material. He was the first to discover the process of radioactivity.
Uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium also occurs in seawater and can be recovered from ocean water. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.
Natural uranium consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). Differences in the half-lives cause the abundance of isotopes in nature. All three naturally occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U).
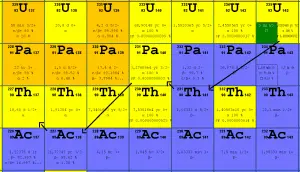
In nuclear reactors we have to consider three artificial isotopes, 236U, 233U and 232U. These are produced by transmutation in nuclear reactors from 235U and 232Th.
Isotopes of Uranium
The main isotopes, which have to be considered in the fuel cycle of all commercial light water reactors, are:
Naturally-occurring isotopes
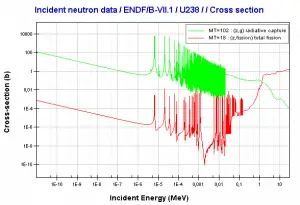
- 238U. 238U belongs to the group of fertile isotopes. 238U decays via alpha decay to 234Th with a half-life of ~4.5×109 years. 238U occasionally decays by spontaneous fission with the probability of 0.000055%. Its specific activity is very low ~3.4×10-7 Ci/g.
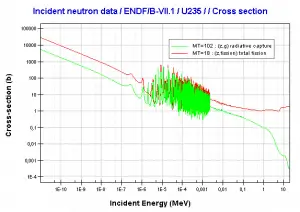
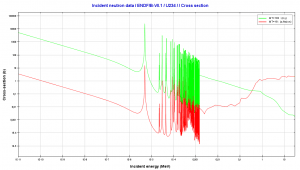
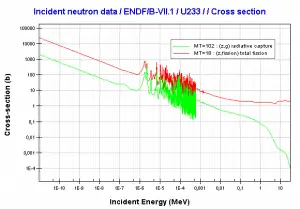
- 235U. 235U belongs to the group of fissile isotopes. 235U is the only existing fissile nucleus from naturally occurring isotopes, and therefore it is a highly strategic material. 235U decays via alpha decay (thorium-231) into 231Pa with a half-life of ~7×108 years. 235U occasionally decays by spontaneous fission with a very low probability of 0.0000000072%. Its specific activity is very low ~2.2×10-6 Ci/g.
- 234U. 234U belongs to the group of fertile isotopes. 234U decays via alpha decay to 230Th with a half-life of 246 000 years. 234U occasionally decays by spontaneous fission with a very low probability of 0.0000000017%. Its specific activity is much higher ~0.0063 Ci/g.
Artificial isotopes
- 233U. 233U belongs to the group of fissile isotopes. It is produced by radiative neutron capture in nuclear reactors containing thorium fuel. 233U decays via alpha decay into 229Th with a half-life of 159 200 years. 233U occasionally decays by spontaneous fission with a very low probability of 0.000000006%. Its specific activity is ~0.0098 Ci/g.
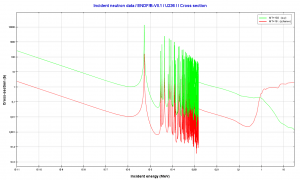
- 236U. 236U is neither a fissile isotope nor a fertile isotope. 236U is fissionable only by fast neutrons. Isotope 236U is formed in a nuclear reactor from fissile isotope 235U. 236U decays via alpha decay to 232Th with a half-life of ~2.3×107 years. 236U occasionally decays by spontaneous fission with a very low probability of 0.00000009%. Its specific activity is ~6.5×10-5 Ci/g.
- 232U. 232U belongs to the group of fertile isotopes. 232U is a side product in the thorium fuel cycle, and also this isotope is a decay product of 236Pu in the uranium fuel. 232U decays via alpha decay to 228Th with a half-life of 68.9 years. 232U very rarely decays by spontaneous fission. Its specific activity is very high, ~22 Ci/g, and its decay chain produces very penetrating gamma rays.
Uranium in the Environment
All three naturally occurring uranium isotopes (238U, 235U, and 234U) have a very long half-life (e.g.,, 4.47×109 years for 238U). Because of this very long half-life, uranium is weakly radioactive and contributes to low natural background radiation levels in the environment. These isotopes are alpha radioactive (emitting alpha particle), but they can also rarely spontaneously fission.
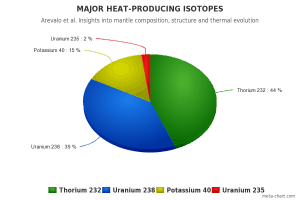
All naturally occurring isotopes belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.54×109 years). Uranium has the second-highest atomic mass of these primordial nuclides, lighter only than plutonium. Moreover, the decay heat of uranium and its decay products (e.g.,, radon, radium, etc.) contributes to heating the Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.
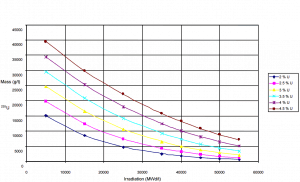
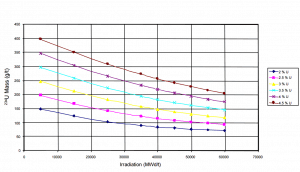
Uranium consumption in a nuclear reactor
Consumption of a 3000MWth (~1000MWe) reactor (12-months fuel cycle)
It is an illustrative example, and the following data do not correspond to any reactor design.
- A typical reactor may contain about 165 tonnes of fuel (including structural material)
- A typical reactor may contain about 100 tonnes of enriched uranium (i.e., about 113 tonnes of uranium dioxide).
- This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods.
- A common fuel assembly contains energy for approximately 4 years of operation at full power.
- Therefore about one-quarter of the core is yearly removed to the spent fuel pool (i.e., about 40 fuel assemblies). At the same time, the remainder is rearranged to a location in the core better suited to its remaining level of enrichment (see Power Distribution).
- The removed fuel (spent nuclear fuel) still contains about 96% of reusable material (it must be removed due to decreasing kinf of an assembly).
- This reactor’s annual natural uranium consumption is about 250 tons of natural uranium (to produce about 25 tons of enriched uranium).
- The annual enriched uranium consumption of this reactor is about 25 tonnes of enriched uranium.
- The annual fissile material consumption of this reactor is about 1 005 kg.
- The annual matter consumption of this reactor is about 1.051 kg.
- But it corresponds to about 3 200 000 tons of coal burned in coal-fired power plant per year.
See also: Fuel Consumption