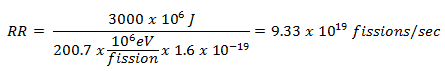IAEA/NVS/3
Uranium consumption in a nuclear reactor
A typical thermal reactor contains about 100 tons of uranium with an average enrichment of 2% (do not confuse it with the enrichment of the fresh fuel, that is about 4%). For the reactor of power of 3000MWth determine the consumption of 235U that must undergo fission each day to provide this thermal power.
Solution:
This problem can be solved very simply. The average recoverable energy per 235U fission is about Er = 200.7 MeV/fission. Since we know, we need 3000 MJ of energy that each second, the required reaction rate can be determined directly as:
Since each atom of 235U has a mass of 235u x 1.66 x 10-27 kg/u = 3.9 x 10-25 kg, the daily consumption of a reactor is:
9.33 x 1019 fissions/sec x 3.9 x 10-25 kg x 86400 sec/day = 3.14 kg/day
For comparison, a 1000 MWe coal-fired power plant burns about 10 000 tons (about 10 million kg) of coal per day.
Since a typical fuel cycle takes about 320 days (12 month fuel cycle), the annual fuel consumption is about:
3.14 kg/day x 320 days = 1 005 kg of 235U