The protons and neutrons in an atomic nucleus are held together by the nuclear forces (strong force). The mass of a nucleus is always less than the sum of masses of the constituent protons and neutrons when separated. The difference is a measure of the nuclear binding energy (Eb) which holds the nucleus together. According to the Einstein relationship (E=m.c2) this binding energy is proportional to this mass difference, known as the mass defect.
Source: hyperphysics.phy-astr.gsu.edu
During the nuclear splitting or nuclear fusion, some of the mass of the nucleus gets converted into huge amounts of energy. Thus this mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. The nuclear binding energies are enormous. They are on the order of a million times greater than the electron binding energies of atoms.
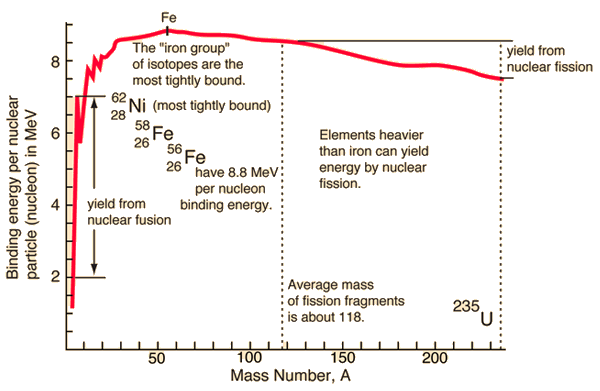
For a nucleus with A (mass number) nucleons, the binding energy per nucleon Eb/A can be calculated. This calculated fraction is shown in the chart as a function of the mass number A. As can be seen, for low mass numbers, Eb/A increases rapidly and reaches a maximum of 8.8 MeV at approximately A=60. The nuclei with the highest binding energies that are most tightly bound belong to the “iron group” of isotopes (56Fe, 58Fe, 62Ni). After that, the binding energy per nucleon decreases. A more stable configuration is obtained in the heavy nuclei (A>60) region when a heavy nucleus splits into two lighter nuclei. This is the origin of the fission process. It may seem that all the heavy nuclei may undergo fission or even spontaneous fission. In fact, for all nuclei with atomic number greater than about 60, fission occurs very rarely. For the fission process to take place, a sufficient amount of energy must be added to the nucleus, no matter how. The energetics and binding energies of the certain nucleus are well described by the Liquid Drop Model, which examines the global properties of nuclei.
See also: Nuclear Binding Curve