Cadmium is a naturally-occurring chemical element with atomic number 48 which means there are 48 protons and 48 electrons in the atomic structure. The chemical symbol for cadmium is Cd. Cadmium was first discovered in 1817 by two German chemists – Friedrich Stromeyer and Karl Samuel Leberecht Hermann.
Natural cadmium consists of eight isotopes, 106Cd (1.3%), 108Cd (0.9%), 110Cd (12.5%), 111Cd (12.8%), 112Cd (24.3%), 113Cd (12.2%), 114Cd (28.7%) and 116Cd (7.5%). Two of them are radioactive isotopes with a very long half-life (113Cd – 7.7 x 1015 y and 116Cd – 2.9 x 1019 y).
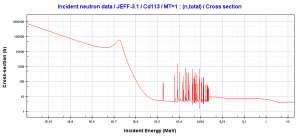
In the nuclear industry, cadmium is commonly used as a thermal neutron absorber due to a very high neutron absorption cross-section of 113Cd. 113Cd has a specific absorption cross-section. There is a cadmium cut-off energy (Cadmium edge) in the absorption cross-section. Only neutrons of kinetic energy below the cadmium cut-off energy (~0.5 eV) are strongly absorbed by 113Cd. Therefore cadmium is widely used to absorb thermal neutrons in thermal neutron filters.