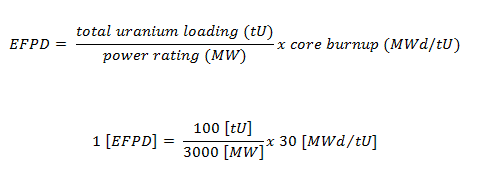
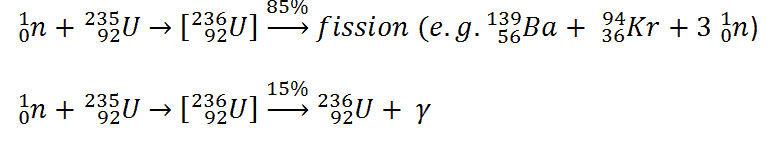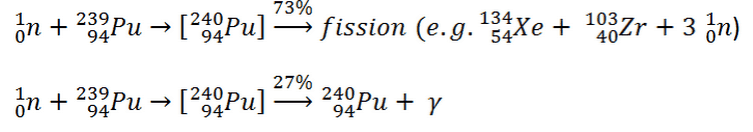Fuel burnup of nuclear fuel normally has units of megawatt-days per metric tonne (MWd/MTU), where tonne refers to a metric ton of uranium metal (sometimes MWd/tU HM as Heavy Metal). For example – BUcore = 25 000 MWd/tHM
Fuel burnup (also known as fuel utilization) measures how much energy is extracted from nuclear fuel and measures fuel depletion in nuclear engineering. The most commonly defined as the fission energy release per unit mass of fuel in megawatt-days per metric ton of heavy metal of uranium (MWd/tHM) or similar units. Fuel burnup defines energy release as well as defines the isotopic composition of irradiated fuel. Since during refueling, every 12 to 18 months, some of the fuel – usually one third or one-quarter of the core – is replaced by fresh fuel assemblies, and power distribution is not uniform in the core, reactor engineers distinguish between:
- Core Burnup. Averaged burnup over entire core (i.e., over all fuel assemblies). For example – BUcore = 25 000 MWd/tHM
- Fuel Assembly Burnup. Averaged burnup over single assembly (i.e., overall fuel pins of a single fuel assembly). For example – BUFA = 40 000 MWd/tHM
- Pin Burnup. Averaged burnup over a single fuel pin or fuel rod (overall fuel pellets of a single fuel pin). For example – BUpin = 45 000 MWd/tHM
- Local or Fine Mesh Burnup. Burnup significantly varies also within a single fuel pellet. For example, the local burnup at the rim of the UO2 pellet can be 2–3 times higher than the average pellet burnup. This local anomaly causes the formation of a structure known as a High Burnup Structure.
Units of Fuel Burnup
There are three different units in common use for describing the state or depletion of the fuel. These units are:
Fuel Depletion – Isotopic Changes
As a reactor is operated at significant power, fuel atoms are constantly consumed, resulting in the slow depletion of the fuel. It must be noted there are also research reactors, which have very low power, and the fuel in these reactors does not change its isotopic composition.
Research reactors with significant thermal power and all power reactors are subjected to significant isotopic changes. The study of these isotopic changes is known as long-term kinetics, which describes phenomena that occur over months or even years. The study of phenomena that occur for several hours to a few days, for example, effects of neutron poisons on the reactivity (i.e., Xenon poisoning or spatial oscillations), is known as medium-term kinetics.
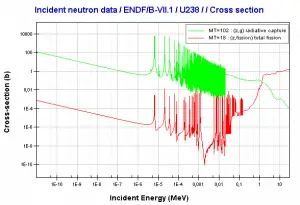
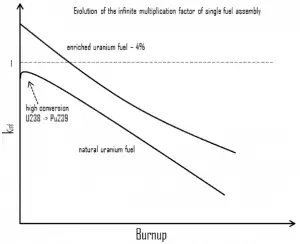
This chapter describes the long-term kinetics of thermal reactors based on the uranium fuel cycle, in which a fuel with a large concentration of uranium-238 is used (e.g., PWRs or BWRs). Most common reactor fuels are composed of either natural or partially enriched uranium. Typically, PWRs use enriched uranium fuel (~4% of U-235) as fresh fuel. Exposure to neutron flux gradually depletes the uranium-235, decreasing core reactivity (compensated by control rods, chemical shim, or burnable absorbers). The initial fuel load of a new reactor core (so-called first core) is entirely fresh fuel, fuel with no plutonium or fission products present. The contribution of uranium-238 directly to fission is quite small in most thermal reactors. On the other hand, uranium-238 plays a very important role, and this chapter is primarily about this isotope.
See also: Uranium
See also: Plutonium
Nuclear Transmutation
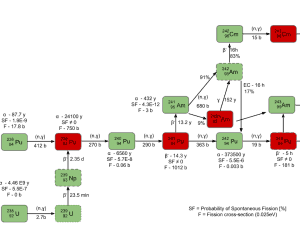 In physics, nuclear transmutation is the conversion of one chemical element or an isotope into another. In nuclear engineering, also nuclear reactors cause artificial transmutation by exposing elements to neutrons produced by fission. In nuclear reactors, the most important phenomenon is the transmutation of fertile isotopes (e.g., uranium 238) into fissile isotopes (e.g., plutonium 239). The process of transmutation of fertile materials to fissile materials is referred to as fuel breeding.
In physics, nuclear transmutation is the conversion of one chemical element or an isotope into another. In nuclear engineering, also nuclear reactors cause artificial transmutation by exposing elements to neutrons produced by fission. In nuclear reactors, the most important phenomenon is the transmutation of fertile isotopes (e.g., uranium 238) into fissile isotopes (e.g., plutonium 239). The process of transmutation of fertile materials to fissile materials is referred to as fuel breeding.
Fertile materials are not capable of undergoing fission reaction after absorbing thermal (slow or low energy) neutrons, and these materials are not capable of sustaining a nuclear fission chain reaction. There are two basic fertile materials: 238U and 232Th.
233U breeding:
232Th is the predominant isotope of natural thorium. If this fertile material is loaded in the nuclear reactor, the nuclei of 232Th absorb a neutron and become nuclei of 233Th. The half-life of 233Th is approximately 21.8 minutes. 233Th decays (negative beta decay) to 233Pa (protactinium), whose half-life is 26.97 days. 233Pa decays (negative beta decay) to 233U, a very good fissile material. On the other hand, proposed reactor designs must attempt to physically isolate the protactinium from further neutron capture before beta decay can occur.
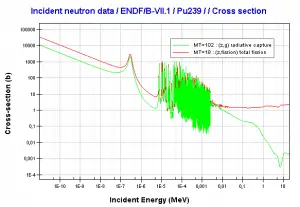
239Pu breeding:
Neutron capture may also be used to create fissile 239Pu from 238U, the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.
Transmutation of transuranium elements (i.e., the chemical elements with atomic numbers greater than 92 ) such as the isotopes of plutonium can help solve some problems posed by the management of radioactive waste by reducing the proportion of long-lived isotopes it contains.
Evolution Equations
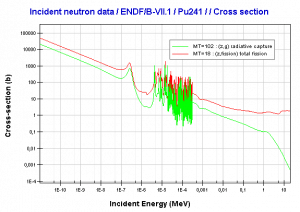
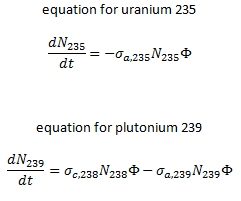
The same evolution of isotopic changes is usually modeled mathematically a set of differential equations known as evolution equations. These equations describe the rate of burnup of U-235, the rate of buildup of Pu-239, production of Pu-240 and Pu-241, the buildup of neutron-absorbing fission products and the overall rate of reactivity change in the reactor due to the changing composition of the fuel. The evolution equation can be constructed for each isotope. For example:
Special reference: W. M. Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-1.
Special reference: Paul Reuss, Neutron Physics, EDP Sciences, 2008, ISBN: 2759800415.
Isotopic Changes – Summary

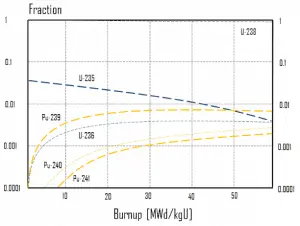
In summary, it can be seen for fuel burnup of 40 GWd/tU:
- Approximately 3 – 4% of the heavy nuclei are fissioned.
- About two-thirds of these fissions come directly from uranium 235, and the other third comes from plutonium produced from uranium 238. The contribution significantly increases as the fuel burnup increases.
- The removed fuel (spent nuclear fuel) still contains about 96% reusable material. It must be removed due to decreasing kinf of an assembly, or in other words, it must be removed due to accumulation of fission products with significant absorption cross-section.
- Discharged fuel contains about 0.8% of plutonium and about 1% of uranium 235. It must be noted there is a significant content (about 0.5%) of uranium 236, which is neither a fissile isotope nor a fertile isotope.
Neutron Poisons – Reactor Poisoning
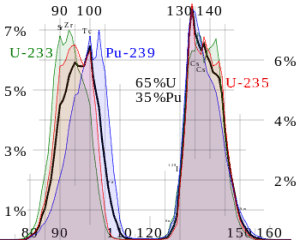
Nuclear fission fragments are the fragments left after a nucleus fissions. The average of the fragment mass is about 118, but very few fragments near that average are found. It is much more probable to break up into unequal fragments, and the most probable fragment masses are around mass 95 (Krypton) and 137 (Barium). Most of these fission fragments are highly unstable (radioactive) and undergo further radioactive decays to stabilize themselves.
Plenty of isotopes, usually neutron-rich isotopes, are produced by fission. Many of the resulting fission products have measurable thermal absorption cross-sections. Fission products are of concern in reactors primarily because they become parasitic absorbers of neutrons and result in long-term sources of heat (so-called decay heat). Their buildup in the reactor tends to reduce the multiplication factor. For that reason, these nuclei are known as neutron poisons.
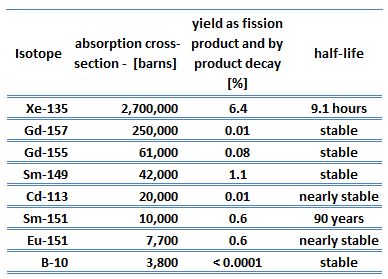
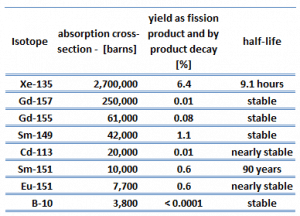
Each poison’s significance depends on the yield, decay rates, and absorption cross-section. Equilibrium is eventually reached during reactor operation if a radioisotope is produced constantly. After several half-lives, the rate of decay will equal the production rate. Most fission products have a low absorption cross-section, but there are very important exceptions. These isotopes are described in the following table.
As can be seen, most of these isotopes are stable, and some undergo decay. From this point of view, we distinguish between neutron poisons, which causes reactor poisoning, and neutron poisons, which cause reactor slagging.
The isotope with a short half-life (e.g., xenon-135) is known as reactor poison, while the isotope that is long-lived or even stable is known as reactor slag. The neutron-absorbing fission products xenon-135 and samarium-149 have particular operational importance.
Their concentrations can change quickly, producing major changes in neutron absorption on a relatively short time scale. Because these two fission product poisons remove neutrons from the reactor, they will impact the thermal utilization factor and thus keff and reactivity. The importance of xenon-135 and samarium-149 is so high that they will be discussed separately.
It must be noted that neutron-absorbing materials, also called neutron poisons, are intentionally inserted into some types of reactors to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant.
Reactor Slagging – Reactor Slag
As was written, an isotope long-lived or even stable is known as reactor slag. The accumulation of these parasitic absorbers is known as reactor slagging and determines the lifetime of nuclear fuel in a reactor. Their buildup causes (together with a decrease in fissile material) a continuous decrease in core reactivity.
 Samarium 149 belongs to this group of isotopes, but its importance is so high that it is usually discussed separately. As can be seen from the table, there are numerous other fission products that, due to their concentration and thermal neutron absorption cross-section, have a poisoning effect on reactor operation. Other fission products with relatively high absorption cross-sections include 83Kr, 95Mo, 143Nd, 147Pm. Individually, they are of little consequence but taken together, and they have a significant effect. These are often characterized as lumped fission product poisons.
Samarium 149 belongs to this group of isotopes, but its importance is so high that it is usually discussed separately. As can be seen from the table, there are numerous other fission products that, due to their concentration and thermal neutron absorption cross-section, have a poisoning effect on reactor operation. Other fission products with relatively high absorption cross-sections include 83Kr, 95Mo, 143Nd, 147Pm. Individually, they are of little consequence but taken together, and they have a significant effect. These are often characterized as lumped fission product poisons.
As was written, for fuel burnup of 40 GWd/tU, approximately 3 – 4% of the heavy nuclei are fissioned. The discharged fuel (spent nuclear fuel) still contains about 96% reusable material. It must be removed due to decreasing kinf of an assembly or in or in other words, and it must be removed due to:
- accumulation of fission products with significant absorption cross-section.
- decrease in fissile material
This is the reason why some nuclear power plant operators use reprocessed fuel to replace fresh uranium fuel partially. Nuclear reprocessing is the technology that was developed to chemically separate and recover fissionable material from spent nuclear fuel as well as to remove these isotopes with significant absorption cross-section.
Effect of Fuel Depletion on the Power Distribution
During the operation of a reactor, the amount of fissile material contained in the fuel assembly constantly decreases; therefore, the assembly kinf constantly decreases. During fuel depletion, the decrease of the assembly kinf will be greatest where the power is greatest. The differences in kinf between fresh fuel assemblies and high-burnup assemblies decreases. Therefore during cycle depletion, this process will cause the power to shift away from regions with the highest kinf. This process also depends on the use of burnable absorbers, which disrupt the first assumption about the constantly decreasing assembly kinf.
Effect of Fuel Depletion on Delayed Neutrons
In LWRs, the delayed neutron fraction decreases with fuel burnup. This is due to isotopic changes in the fuel. It is simple, and fresh uranium fuel contains only 235U as the fissile material, meanwhile during fuel burnup, the importance of fission of 239Pu increases (in some cases up to 50%). Since 239Pu produces significantly less delayed neutrons (0.0021 vs. 0.0064), the resultant core delayed neutron fraction of a multiplying system decreases (the weighted average of the constituent delayed neutron fractions). It follows then that the amount of reactivity insertion needed to produce a given reactor period decreases with the burnup of the fuel. This is also the reason why the neutron spectrum in the core becomes harder with fuel burnup.
βcore= ∑ Pi.βi
where Pi is the fraction of power generated by isotope i.
Reactivity Effects of Fuel Burnup
 ↑burnup ⇒ ↓keff = ↓η .ε.p. ↓f . ↑↓Pf. ↑↓Pt
↑burnup ⇒ ↓keff = ↓η .ε.p. ↓f . ↑↓Pf. ↑↓Pt
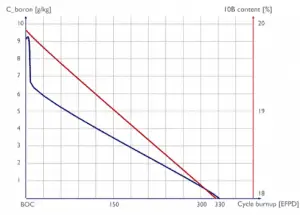
It is hard to describe the effects of fuel burnup on the six-factor formula, and it must be noted that criticality must be maintained for a long period. Therefore all the negative effects must be compensated by increasing the thermal utilization factor (boron dilution or compensating rods withdrawal).
See more: Operational factors that affect the multiplication
Neutron Flux Effects of Fuel Burnup
In a power reactor over a relatively short period (days or weeks), the atomic number density of the fuel atoms remains relatively constant. Therefore, the average neutron flux remains constant in this short period when the reactor is operated at a constant power level. On the other hand, the atomic number densities of fissile isotopes for months decrease due to the fuel burnup, and the macroscopic cross-sections decrease. Note that the thermal power is determined by reaction rate (RR = Ф . Σ), in which Σ is continuously decreasing. This result is slow to increase the neutron flux to keep the desired power level.
LWR with High Burnup Fuel
Current light water reactors are typically designed to achieve burnup of about 50 GWd/tU. With newer fuel technology, and particularly the use of advanced burnable absorbers, these same reactors can achieve up to 60 GWd/tU. Some studies show that soon, even with the present enrichment limit (5 wt %), fuel burnup could be extended near to 70 MWd/kg. Further increase in fuel burnup is impossible without the relaxation of the present enrichment limit (5%).
As can be seen, there has been a clear trend over the last decades toward increasing fuel burnup in light water reactors. In nuclear power plants, high fuel burnup is desirable for:
- Reducing the number of fresh nuclear fuel assemblies required and thus reducing spent nuclear fuel assemblies generated. Both aspects lead to improvements in the economics of the fuel cycle.
- Reducing the duration of refueling outage.
- High burnup results in a lower mass of spent fuel discharged per unit of electricity generated, reducing spent fuel handling and transportation.
- A benefit (crucial aspect for some operators) is that loading the “high” burnup assemblies in the periphery reduces the neutron flux on the pressure vessel.
- Reducing the potential for diversion of fissile material from spent fuel for non-peaceful.
On the other hand, some signals increasing burnup above 50 or 60 GWd/tU leads to significant engineering challenges, and even it does not necessarily have to lead to economic benefits.
See also: Etienne Parent. Nuclear Fuel Cycles for Mid-Century Deployment. MIT. 2009.
Higher burnup fuels require higher initial enrichment to sustain reactivity. Since the number of separative work units (SWUs) is not a linear enrichment function, it is more expensive to enrich higher enrichments. For instance, to produce one kilogram of uranium enriched to:
- 5% U-235 requires 8.9 SWU if the tails assay is 0.20%
- 4% U-235 requires 6.3 SWU if the tails assay is 0.20%
But there are also operational aspects of high burnup fuels that are associated especially with the reliability of such fuel. The main concerns associated with high burnup of fuels are:
- Increased burnup places additional demands on fuel cladding, which must withstand the reactor environment for a longer period.
- The longer residence in the reactor requires a higher corrosion resistance.
- Higher burnup leads to a higher accumulation of gaseous fission products inside the fuel pin resulting in a significant increase in internal pressure.
- Higher burnup leads to increased radiation-induced growth, leading to undesirable changes in core geometry (fuel assembly bow or fuel rod bow). FA bow can result in an increased control rods drop time due to friction between the control rod and bowed guide tubes.
- These factors must be considered because they can lead to a decrease in operational reliability.
- High burnup fuel generates a smaller fuel volume for the reprocessing but more specific activity.
Strategic management and decision-making regarding the middle part of nuclear fuel cycles is a very specific problem of power engineering. Such evaluation must also contain nuclear calculations and business-economic evaluation; thus, they cannot be carried out separately. Detailed nuclear calculations are necessary because they can exclude many promising strategies. On the other hand, they do not provide any information about benefits or costs. Strategic decision-making must be carried out based on business-economic evaluations.
Special reference: Technical and economic limits to fuel burnup extension, Proceedings of a Technical Committee meeting, IAEA, 11/2009