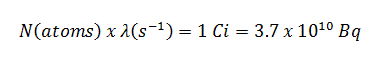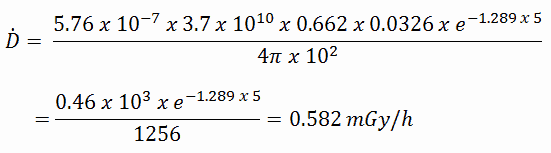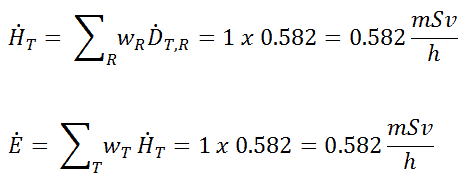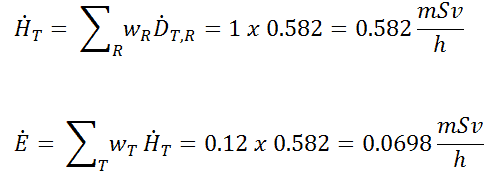Contamination is generally referred to as the presence of an unwanted constituent, harmful substance, or impurity in a location (material, physical body, natural environment, workplace) where it is not intended or desired. Contamination has a much more general meaning since it can be defined in disciplines such as chemistry, environmental protection, radiation protection, or agriculture.
Radioactive contamination is referred to as the presence of unwanted radioactive substances on surfaces or within solids (including the human body), liquids, or gases, where their presence is unintended or undesirable. Radioactive contamination consists of radioactive atoms (material) that have escaped the system or structure that would normally contain them. Since radioactive contamination is radioactive material, ionizing radiation is emitted by the contamination. It is very important which material (radioisotope) is the radioactive contaminant, and it is also very important to distinguish between radioactive contamination and radiation.
Contamination versus Radiation
Radioactive contamination consists of radioactive material that generates ionizing radiation and is the source of radiation, not radiation itself. Anytime that radioactive material is not in a sealed radioactive source container and might be spread onto other objects, radioactive contamination is possible. Radioactive contamination may be characterized by the following points:
- Radioactive contamination consists of radioactive material (contaminants) that may be solid, liquid, or gaseous. Large contaminants can be visible, but you cannot see radiation produced.
- When released, contaminants can be spread by air, water, or mechanical contact.
- We cannot shield against contamination.
- We can mitigate contamination by protecting the integrity of barriers (source container, fuel cladding, reactor vessel, containment building)
- Since contaminants interact chemically, they may be contained within objects such as the human body.
- We can eliminate contamination by many mechanical, chemical (decontaminate surfaces), or biological processes (biological half-life).
- It is of the highest importance which material is the radioactive contaminant (half-life, mode of decay, energy).
Ionizing radiation is formed by high-energy particles (photons, electrons, etc.) that can penetrate matter and ionize (to form ions by losing electrons) target atoms to form ions. Radiation exposure is the consequence of the presence nearby the source of radiation. Radiation exposure as a quantity is defined as a measure of the ionization of material due to ionizing radiation. The danger of ionizing radiation lies in the fact that the radiation is invisible and not directly detectable by human senses. People can neither see nor feel radiation, yet it deposits energy into the molecules of the body. The energy is transferred in small quantities for each interaction between radiation and a molecule, and there are usually many such interactions. Unlike radioactive contamination, radiation may be characterized by the following points:
- Radiation consists of high-energy particles that can penetrate matter and ionize (to form ions by losing electrons) target atoms. Radiation is invisible and not directly detectable by human senses. It must be noted that beta radiation is indirectly visible due to Cherenkov radiation.
- Unlike contamination, radiation cannot be spread by any medium. It travels through materials until it loses its energy. We can shield radiation (e.g., by standing around the corner).
- Exposure to ionizing does not necessarily mean that the object becomes radioactive (except for very rare neutron radiation).
- Radiation can penetrate barriers, but a sufficiently thick barrier can minimize all effects.
- Unlike contaminants, radiation cannot interact chemically with matter and cannot be bound inside the body.
- It is not important which material is the source of certain radiation. The only type of radiation and energy matters.
One common feature is natural radiation, and natural contaminants are all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us, and it is a part of our natural world that has been here since the birth of our planet. From the beginning of time, all living creatures have been, and are still being, exposed to ionizing radiation. Natural background radiation is ionizing radiation that originates from various natural sources. From the beginning of time, all living creatures have been, and are still being, exposed to ionizing radiation. This radiation is not associated with any human activity. There are radioactive isotopes in our bodies, houses, air, water, and soil. We all are also exposed to radiation from outer space.
Types of Contamination
Radioactive materials may exist on surfaces or in volumes of material or air, and special techniques are used to measure contamination levels by detecting the emitted radiation. We can distinguish between the following types of contamination:
Surface Contamination
Surface contamination means that radioactive material has been deposited on surfaces (such as walls and floors). It may be loosely deposited, much like ordinary dust, or quite firmly fixed by a chemical reaction. This distinction is important, and we classify surface contamination based on how easily it can be removed:
- Free Contamination. In the case of free contamination (or loose contamination), the radioactive material can be spread. This is surface contamination that can easily be removed with simple decontamination methods. For example, if dust particles containing various radioisotopes land on the person’s skin or garments, we can clean it up or remove clothes. Once a person has decontaminated, all particulate radioactivity sources are eliminated, and the individual is no longer contaminated. Free contamination is also a more serious hazard than fixed contamination because dust particles can become airborne and easily ingested, leading to internal exposure to radioactive contaminants. Although almost all contaminants are beta radioactive with accompanying gamma emission, alpha contamination is also possible in any nuclear fuel handling area.
- Fixed Contamination. In the case of fixed contamination, the radioactive material cannot be spread since it is chemically or mechanically bound to structures. It cannot be removed by normal cleaning methods. Fixed contamination is a less serious hazard than free contamination and cannot be re-suspended or transferred to the skin. Therefore the hazard is usually an external one only. On the other hand, it depends on the level of contamination. Although almost all contaminants are beta radioactive with accompanying gamma emission, there is also the possibility of alpha contamination in any nuclear fuel handling areas. Unless the level of contamination is very severe, the gamma radiation dose rate will be small and external exposure will be significant only in contact with, or very close to, the contaminated surfaces. Since beta particles are less penetrating than gamma rays, the beta dose rate can be high only at contact. A value of 1 mSv/h at contact for a contamination level of 400 – 500 Bq/cm2 is fairly representative.
Airborne Contamination
This type of contamination is particularly important in nuclear power plants, which must be monitored. Contaminants can become airborne, especially during reactor top head removal, reactor refueling, and during manipulations within the spent fuel pool. The air can be contaminated with radioactive isotopes, especially particulate form, which poses a particular inhalation hazard. This contamination consists of various fission and activation products that enter the air in gaseous, vapor, or particulate form. There are four types of airborne contamination in nuclear power plants, namely:
- Particulates. Particulate activity is an internal hazard because it can be inhaled. Transportable particulate material taken into the respiratory system will enter the bloodstream and be carried to all body parts. Non-transportable particulates will stay in the lungs with a certain biological half-life. For example, Sr-90, Ra-226, and Pu-239 are radionuclides known as bone-seeking radionuclides. These radionuclides have long biological half-lives and are serious internal hazards. Once deposited in bone, they remain unchanged in amount during the individual’s lifetime. The continued action of the emitted alpha particles can cause significant injury: over many years, they deposit all their energy in a tiny volume of tissue because the range of the alpha particles is very short.
- Noble gases. Radioactive noble gases, such as xenon-133, xenon-135, and krypton-85, are present in reactor coolant, especially when fuel leakages are present. As they appear in coolant, they become airborne and can be inhaled, and they are exhaled right after inhaling because the body does not react chemically with them. If workers work in a noble gas cloud, the external dose they will receive is about 1000 times greater than the internal dose. Because of this, we are only concerned about the external beta and gamma dose rates.
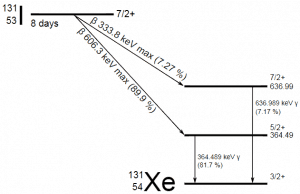 Radioiodine. Radioiodine, iodine-131, is an important radioisotope of iodine. Radioiodine plays a major role as a radioactive isotope present in nuclear fission products. It is a major contributor to health hazards when released into the atmosphere during an accident. Iodine-131 has a half-life of 8.02 days. The target tissue for radioiodine exposure is the thyroid gland. The external beta and gamma dose from radioiodine present in the air is quite negligible compared to the committed dose to the thyroid resulting from breathing this air. The biological half-life for iodine inside the human body is about 80 days (according to ICRP). Iodine in food is absorbed by the body and preferentially concentrated in the thyroid, where it is needed for the functioning of that gland. When 131I is present in high levels in the environment from radioactive fallout, it can be absorbed through contaminated food and accumulate in the thyroid. 131I decays with a half-life of 8.02 days with beta particle and gamma emissions. As it decays, it may cause damage to the thyroid. The primary risk from exposure to high levels of 131I is the chance of occurrence of radiogenic thyroid cancer in later life. For 131I, ICRP has calculated that if you inhale 1 x 106 Bq, you will receive a thyroid dose of HT = 400 mSv (and a weighted whole-body dose of 20 mSv).
Radioiodine. Radioiodine, iodine-131, is an important radioisotope of iodine. Radioiodine plays a major role as a radioactive isotope present in nuclear fission products. It is a major contributor to health hazards when released into the atmosphere during an accident. Iodine-131 has a half-life of 8.02 days. The target tissue for radioiodine exposure is the thyroid gland. The external beta and gamma dose from radioiodine present in the air is quite negligible compared to the committed dose to the thyroid resulting from breathing this air. The biological half-life for iodine inside the human body is about 80 days (according to ICRP). Iodine in food is absorbed by the body and preferentially concentrated in the thyroid, where it is needed for the functioning of that gland. When 131I is present in high levels in the environment from radioactive fallout, it can be absorbed through contaminated food and accumulate in the thyroid. 131I decays with a half-life of 8.02 days with beta particle and gamma emissions. As it decays, it may cause damage to the thyroid. The primary risk from exposure to high levels of 131I is the chance of occurrence of radiogenic thyroid cancer in later life. For 131I, ICRP has calculated that if you inhale 1 x 106 Bq, you will receive a thyroid dose of HT = 400 mSv (and a weighted whole-body dose of 20 mSv).- Tritium. Tritium is a byproduct of nuclear reactors. The most important source (due to releases of tritiated water) of tritium in nuclear power plants stems from the boric acid, commonly used as a chemical shim to compensate for an excess of initial reactivity. Note that tritium emits low-energy beta particles with a short-range in body tissues and, therefore, poses a risk to health as a result of internal exposure only following ingestion in drinking water or food, or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. According to the ICRP, a biological half-time of tritium is 10 days for HTO and 40 days for OBT (organically bound tritium) formed from HTO in the body of adults. As a result, for an intake of 1 x 109 Bq of tritium (HTO), an individual will get a whole-body dose of 20 mSv (equal to the intake of 1 x 106 Bq of 131I). While for PWRs, tritium poses a minor risk to health, for heavy water reactors, it contributes significantly to a collective dose of plant workers. Note that “Air saturated with moderator water at 35°C can give 3 000 mSv/h of tritium to an unprotected worker (See also: J.U.Burnham. Radiation Protection). The best protection from tritium can be achieved using an air-supplied respirator. Tritium cartridge respirators protect workers only by a factor of 3. The only way to reduce skin absorption is by wearing plastics. In PHWR power plants, workers must wear plastics for work in atmospheres containing more than 500 μSv/h.
Respirators with suitable air filters or completely self-contained suits with their air supply can mitigate dangers from airborne contamination. Airborne contamination is usually measured by special radiological instruments that continuously pump the sampled air through a filter. Instruments that do this are called Continuous Air Monitors (CAM). Radioactive particulates in the air collect on the filter, where the activity is measured by a detector placed close to the filter.
See also: Derived Air Concentration
See also: Annual Limit on Intake
Decontamination
Decontamination is a process used to reduce or remove radioactive contamination to reduce the risk of radiation exposure. Equipment and personnel are important to maintain an ionizing radiation dose as low as reasonably achievable (ALARA), removing contamination from occupied areas. Decontamination also reduces background radiation levels, radioactive material inventory, and the spread of contamination to uncontrolled areas, equipment, and personnel.
Decontamination may be accomplished by cleaning or treating surfaces to reduce or remove the contamination. It may also be accomplished by filtering contaminated air or water or covering the contamination to shield or absorb the radiation. The process allows adequate time for natural radioactive decay to decrease radioactivity.
In nuclear power plants, many items of equipment, tools, clothing, work areas, and even people will inevitably become contaminated. This is quite common that some radioactive material becomes attached to surfaces (e.g., the sole of a shoe). In this case, workers are continuously monitored, and in this case, surface contamination must be removed. We can eliminate contamination from many mechanical and chemical (decontaminate surfaces). Biological processes (biological half-life) always work in case of internal contamination. A person becomes ‘radioactive’ if dust particles containing various radioisotopes land on the person’s skin or garments. Once a person has been decontaminated by clothes removal and dermal scrubbing, all particulate radioactivity sources are eliminated, and the individual is no longer contaminated.
Decontamination Techniques
In general, many techniques and equipment are used to decontaminate surfaces and persons. In any case, the type of contamination and contaminated material matters. For example, it isn’t easy to decontaminate porous materials. As a general orientation to the reader, these decontamination techniques and their main applications are highlighted in:
Special Reference: State of the Art Technology for Decontamination and Dismantling of Nuclear Facilities, IAEA. IAEA Vienna, 1999. ISBN 92–0–102499–1.
- Chemical Decontamination. Chemical decontamination is one of the best methods for most decontamination operations is to clean with water to which one or more suitable chemical cleaning agents have been added. These methods include using chemical solutions, gels, foam decontamination, etc. Removing contamination from personnel must be done carefully to ensure the skin is not damaged and to prevent contamination from entering the body or a wound.
- Mechanical Decontamination. Mechanical decontamination can be used especially for industrial decontamination. There are decontamination methods in which the outer layer of the contaminated surface is removed by physical force. Such methods are effective but somewhat crude and destructive, and it may not be possible to use them on delicate objects. These methods include decontamination using steam cleaning, abrasive cleaning, sandblasting, vacuum cleaning, ultrasonic cleaning, etc.