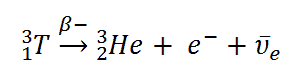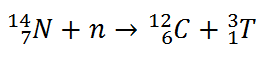Tritium
Tritium is the only naturally occurring radioisotope of hydrogen. Its atomic number is naturally 1, which means there is 1 proton and 1 electron in the atomic structure. Unlike the hydrogen nucleus and deuterium nucleus, tritium has 2 neutrons in the nucleus. Tritium is naturally occurring, but it is extremely rare. Tritium is produced in the atmosphere when cosmic rays collide with air molecules. Tritium is also a byproduct of the production of electricity by nuclear power plants. The name of this isotope is formed from the Greek word τρίτος (trítos), meaning “third”.
Decay of Tritium
Tritium is a radioactive isotope, but it emits a very weak form of radiation, a low-energy beta particle similar to an electron. It is a pure beta emitter (i.e., beta emitter without accompanying gamma radiation). The electron’s kinetic energy varies, with an average of 5.7 keV, while the nearly undetectable electron antineutrino carries off the remaining energy. Such a very low electron energy causes the electron not to penetrate the skin or even travel very far in the air. Beta particles from tritium can penetrate only about 6.0 mm of air.
Tritium decays via negative beta decay into helium-3 with half-life of 12.3 years.
3H
Tritium in nuclear reactors
Tritium is a byproduct of nuclear reactors. The most important source (tritium releases of tritiated water) in nuclear power plants stems from boric acid, commonly used as a chemical shim to compensate for an excess of initial reactivity. The main reactions in which the tritium is generated from boron are below:
10B(n,T + 2*alpha)
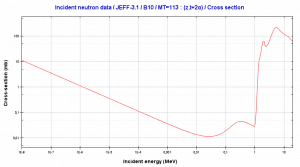
This threshold reaction of the fast neutron with an isotope 10B is the main way radioactive tritium in the primary circuit of all PWRs is generated. 10B is the principal source of radioactive tritium in the primary circuit of all PWRs (which use boric acid as a chemical shim). 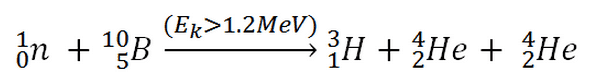 Note that this reaction occurs very rarely compared to the most common (n, alpha) reaction of isotope 10B with thermal neutrons.
Note that this reaction occurs very rarely compared to the most common (n, alpha) reaction of isotope 10B with thermal neutrons.
There are more reactions with neutrons, which can rarely lead to formation of radioactive tritium, for example:
10B(n,alpha)7Li + 7Li(n,n+alpha)3H – threshold reaction (~3 MeV).
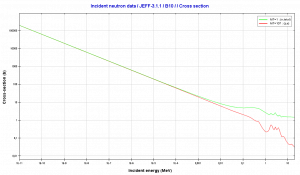 Boron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.
Boron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library[/captionBoron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Tritium is also a fission product (ternary fission) of the splitting of fissionable materials. In fact, fission probably produces more tritium than all other sources in Light Water Reactors. Its production (yield) is about one atom per 10,000 fissions. On the other hand, only a very small fraction of the fission-product tritium diffuses out of the fuel matrix and fuel cladding into the primary coolant.Tritium is also produced in reaction with 6Li.
6Li(n,α)3H
This reaction allows neutrons detection, but in some cases, LiOH is added to control the pH of primary coolant in some LWR. The reaction cross-section for thermal neutrons is σ = 925 barns, and the natural lithium has an abundance of 6Li 7,4%.
Tritium occurs in nuclear power plants in the form of tritiated water. Tritiated water is like normal water but is very weakly radioactive. Therefore it does not pose a hazard to human health. Plant operators and state supervisors closely monitor the releases of tritiated water.
Reference: Jacobs D.G. Sources of Tritium and Its Behaviour Upon Release to the Environment. US Atomic Energy Commission, 1968.
Tritium in Nature
Tritium is produced in the atmosphere when cosmic rays collide with air molecules. In the most important reaction for natural production, a fast neutron (which must have energy greater than 4.0 MeV) interacts with atmospheric nitrogen:
Worldwide, the production of tritium from natural sources is 148 petabecquerels per year. As a result, the tritiated water produced participates in the water cycle.
- about 400 Bq/m3 in continental water
- about 100 Bq/m3 in oceans
Tritium poses a risk to health due to internal exposure only the following ingestion in drinking water or food or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. An average annual dose from natural tritium intake is 0.01 μSv.
In case of artificial tritium ingestion or inhalation, a biological half-time of tritium is 10 days for HTO and 40 days for OBT (organically bound tritium) formed from HTO in the body of adults. It was also shown that the biological half-time of HTO depends strongly on many variables and varies from about 4 to 18 days. During the warmer months, the average half-life is lower, which is attributed to increased water intake. As well as, drinking larger amounts of alcohol will reduce the biological half-life of water in the body.
See also: Tritium in Nature.