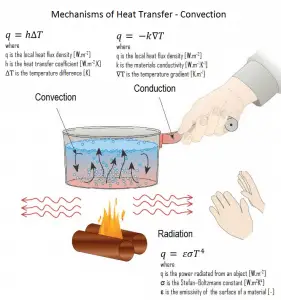
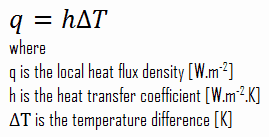In general, convection is either the mass transfer or the heat transfer due to the bulk movement of molecules within fluids such as gases and liquids. Although liquids and gases are generally not very good conductors of heat, they can transfer heat quite rapidly by convection.
Convection takes place through advection, diffusion, or both. Convection cannot occur in most solids because neither significant diffusion of matter nor bulk current flows occur. Diffusion of heat occurs in rigid solids, called thermal conduction.
The process of heat transfer between a surface and a fluid flowing in contact with it is called convective heat transfer. In engineering, convective heat transfer is one of the major mechanisms of heat transfer. When heat is to be transferred from one fluid to another through a barrier, convection is involved on both sides of the barrier. In most cases, the main resistance to heat flow is by convection. Convective heat transfer take place both by thermal diffusion (the random motion of fluid molecules) and by advection, in which matter or heat is transported by the larger-scale motion of currents in the fluid.
Mechanism on Convection
In thermal conduction, energy is transferred as heat either due to the migration of free electrons or lattice vibrational waves (phonons). There is no mass movement in the direction of energy flow, and heat transfer by conduction depends on the driving “force” of temperature difference. Conduction and convection are similar in that both mechanisms require the presence of a material medium (in comparison to thermal radiation). On the other hand, they are different in that convection requires the presence of fluid motion.
At the surface, it must be emphasized that energy flow occurs purely by conduction, even in conduction. It is because there is always a thin stagnant fluid film layer on the heat transfer surface. But in the next layers, both conduction and diffusion-mass movement occur at the molecular or macroscopic levels. Due to the mass movement, the rate of energy transfer is higher. The higher the mass movement rate, the thinner the stagnant fluid film layer will be, and the higher the heat flow rate.
It must be noted nucleate boiling at the surface effectively disrupts this stagnant layer. Therefore, nucleate boiling significantly increases the ability of a surface to transfer thermal energy to the bulk fluid.
As was written, heat transfer through a fluid is by convection in the presence of mass movement and conduction in its absence. Therefore, thermal conduction in a fluid can be viewed as the limiting case of convection, corresponding to the case of quiescent fluid.
Newton’s Law of Cooling
Despite the complexity of convection, the rate of convection heat transfer is observed to be proportional to the temperature difference. It is conveniently expressed by Newton’s law of cooling, which states that:
The rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its surroundings provided the temperature difference is small and the nature of radiating surface remains same.
Note that, ΔT is given by the surface or wall temperature, Twall and the bulk temperature, T∞, which is the temperature of the fluid sufficiently far from the surface.