Article Summary & FAQs
What is heat transfer?
Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering, it determines key parameters and materials of heat exchangers.
Key Facts
- Heat is the amount of energy flowing spontaneously from one body to another due to its temperature difference. Heat is a form of energy, but it is energy in transit.
- Heat transfer is usually classified into various mechanisms, such as:
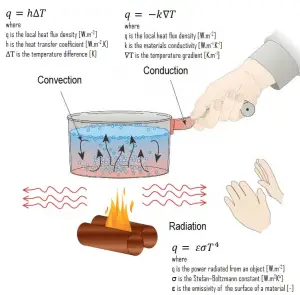
- Heat Conduction. Heat conduction, also called diffusion, occurs within a body or between two bodies in contact. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems.
- Heat Convection. Heat convection depends on the mass motion from one region of space to another. Heat convection occurs when the bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid.
- Thermal Radiation. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need to be present in the space between bodies.
- Fourier’s law of thermal conduction law states that the time rate of heat transfer through a material is proportional to the negative gradient in the temperature and the area at right angles to that gradient, through which the heat flows.
- Newton’s law of cooling states that the rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its surroundings, provided the temperature difference is small and the nature of radiating surface remains the same.
- Stefan–Boltzmann law states that radiation heat transfer rate, q [W/m2], from a body (e.g., a black body) to its surroundings is proportional to the fourth power of the absolute temperature.
- Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common pressures. Therefore large amounts of heat can be transferred during boiling and condensation at a constant temperature.
- Heat exchangers are devices used to transfer thermal energy from one fluid to another without mixing the two fluids.
- To minimize heat losses in industry and also in the construction of buildings, thermal insulation is widely used. The purpose of thermal insulation is to reduce the overall heat transfer coefficient by adding material with low thermal conductivity.
Heat Transfer
 Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering, it determines key parameters and materials of heat exchangers. Heat transfer is usually classified into various mechanisms, such as:
Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering, it determines key parameters and materials of heat exchangers. Heat transfer is usually classified into various mechanisms, such as:
- Heat Conduction. Heat conduction, also called diffusion, occurs within a body or between two bodies in contact. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems. When an object is at a different temperature from another body or its surroundings
- Heat Convection. Heat convection depends on the mass motion from one region of space to another. Heat convection occurs when the bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid.
- Thermal Radiation. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need for the matter to be present in the space between bodies.
Heat Transfer in Nuclear Engineering – Application
Heat transfer is commonly encountered in engineering systems and other aspects of life, and one does not need to go very far to see some application areas of heat transfer.
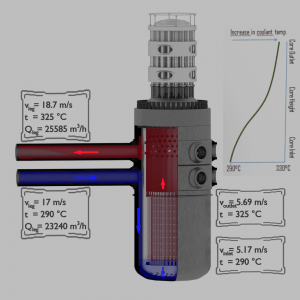
Detailed knowledge of heat transfer mechanisms is also essential for reactor engineers as well as all other engineers. A nuclear power plant (nuclear power station) looks like a standard thermal power station with one exception. The heat source in the nuclear power plant is a nuclear reactor. As is typical in all conventional thermal power stations, the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity. But in nuclear power plants, reactors produce an enormous amount of heat (energy) in a small volume. The density of the energy generation is very large, which puts demands on its heat transfer system (reactor coolant system). Therefore we have to start with the reactor heat generation and removal from the reactor.
For a reactor to operate in a steady-state, all of the heat released in the system must be removed as fast as it is produced. This is accomplished by passing a liquid or gaseous coolant through the core and through other regions where heat is generated. The heat transfer must be equal to or greater than the heat generation rate or overheating, and possible damage to the fuel may occur. The nature and operation of this coolant system are some of the most important considerations in designing a nuclear reactor.
It should be noted that from a strictly nuclear standpoint, there is theoretically no upper limit to the reactor thermal power, which can be attained by any critical reactor having sufficient excess of reactivity to overcome its negative temperature feedbacks. There is a direct proportionality between the neutron flux and thermal power in each nuclear reactor. The term thermal power is usually used because it means the rate at which heat is produced in the reactor core due to fissions in the fuel. Moreover, for a short period, a critical reactor does not need to have high excess of reactivity as in the case of rapid reactivity excursions.
In short, almost any reactor can exceed the ability of heat removal of its coolant system. Beyond this point, the fuel would heat up and reach very high temperatures. This situation must be avoided by reactor operators and by reactor safety systems. The heat generation – heat removal rate balance must be maintained to prevent these temperatures that might fail fuel or other structural materials. In reactor engineering, the thermal-hydraulics of nuclear reactors describe the effort involving the coupling of heat transfer and fluid dynamics to accomplish the desired heat removal rate from the core under both normal operation and accident conditions.