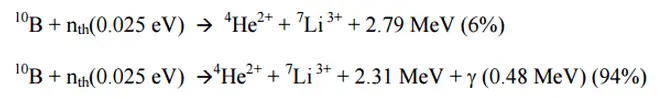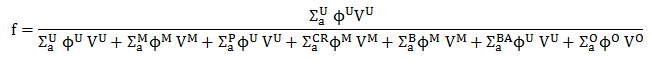The boron coefficient is defined as the change in reactivity per the change in the boron concentration.
αB = dρ⁄dg/kg
It is expressed in units of pcm/g.kg-1. The value of boron coefficient in PWRs is usually ranges from about -1000 pcm/g.kg-1 to about -2000 pcm/g.kg-1. The boron coefficient usually decreases (becomes more negative) as the fuel burnup increases.
This coefficient does not represent any reactivity feedback. Still, it is commonly used by reactor operators because it describes the influence of changes in the boron concentration on the reactor’s reactivity.
See also: Boron 10
Theory of Boron Coefficient
↑boron ⇒ ↓keff = η.ε.p. ↓f .Pf.Pt
The concentration of boric acid diluted in the primary coolant influences the thermal utilization factor. For example, an increase in the concentration of boric acid (chemical shim) causes the addition of new absorbing material into the core, and this causes a decrease in the thermal utilization factor.
The thermal utilization factor for heterogeneous reactor cores must be calculated in terms of reaction rates and volumes, for example, by the following equation:
where Σa is the macroscopic absorption cross-section, which is the sum of the capture cross-section and the fission cross-section, Σa = Σc + Σf. The superscripts U, M, P, CR, B, BA, and O, refer to uranium fuel, moderator, poisons, control rods, boric acid, burnable absorbers, etc. The presence of control rods, boric acid, or poisons causes a decrease in neutron utilization, which, in turn, causes a decrease in the multiplication factor.
Compared with burnable absorbers (long-term reactivity control) or with control rods (rapid reactivity control), the boric acid avoids the unevenness of neutron-flux density in the reactor core because it is dissolved homogeneously in the coolant in the entire reactor core. On the other hand, high concentrations of boric acid may lead to a positive moderator temperature coefficient, which is undesirable. In this case, more burnable absorbers must be used.
Moreover this method is slow in controlling reactivity. Normally, it takes several minutes to change the concentration (dilute or borate) of the boric acid in the primary loop. For rapid changes of reactivity control rods must be used.