There are three main features of neutrons, which are crucial in the shielding of neutrons.
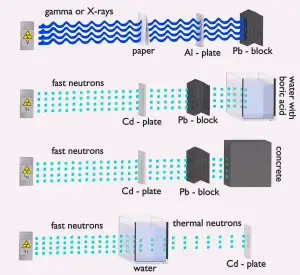 Neutrons have no net electric charge. Therefore they cannot be affected or stopped by electric forces. Neutrons ionize matter only indirectly, which makes neutrons highly penetrating types of radiation.
Neutrons have no net electric charge. Therefore they cannot be affected or stopped by electric forces. Neutrons ionize matter only indirectly, which makes neutrons highly penetrating types of radiation.- Neutrons scatter with heavy nuclei very elastically. Heavy nuclei very hard slow down a neutron, let alone absorb a fast neutron.
- An absorption of neutron (shielding) causes the initiation of certain nuclear reactions (e.g.,, radiative capture or even fission), accompanied by many other types of radiation. In short, neutrons make matter radioactive, and therefore, we have to shield the other types of radiation with neutrons.
See also: Interaction of Neutrons with Matter.
Principles of Neutron Shielding
The best materials for shielding neutrons must be able to:
- Slow down neutrons (the same principle as neutron moderation). The first point can be fulfilled only by a material containing light atoms (e.g.,, hydrogen atoms), such as water, polyethylene, and concrete. The nucleus of a hydrogen nucleus contains only a proton. Since a proton and a neutron have almost identical masses, a neutron scattering on a hydrogen nucleus can give up a great amount of its energy (even the whole kinetic energy of a neutron can be transferred to a proton after one collision). This is similar to a billiard. Since a cue ball and another billiard ball have identical masses, the cue ball hitting another ball can be made to stop, and the other ball will start moving with the same velocity. On the other hand, if a ping pong ball is thrown against a bowling ball (neutron vs. heavy nucleus), the ping pong ball will bounce off with very little change in velocity, only a change in direction. Therefore, lead is quite ineffective for blocking neutron radiation, as neutrons are uncharged and can pass through dense materials.
-
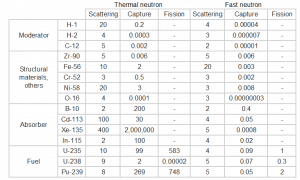
Table of cross-sections Absorb this slow neutron. Thermal neutrons can be easily absorbed by capture in materials with high neutron capture cross-sections (thousands of barns) like boron, lithium, or cadmium. Generally, only a thin layer of such absorber is sufficient to shield thermal neutrons. Hydrogen (in the form of water), which can be used to slow down neutrons, has an absorption cross-section of 0.3 barns. This is not enough, but this insufficiency can be offset by sufficient thickness of the water shield.
- Shield the accompanying radiation. In the case of cadmium shield, the absorption of neutrons is accompanied by strong emission of gamma rays. Therefore additional shield is necessary to attenuate the gamma rays. This phenomenon practically does not exist for lithium and is much less important for boron as a neutron absorption material. For this reason, materials containing boron are often used in neutron shields. In addition, boron (in the form of boric acid) is well soluble in water, making this combination a very effective neutron shield.
Water as a neutron shield
Water, due to the high hydrogen content and availability, is effective and common neutron shielding. However, due to the low atomic number of hydrogen and oxygen, water is not an acceptable shield against gamma rays. On the other hand, in some cases, this disadvantage (low density) can be compensated by the high thickness of the water shield. In the case of neutrons, water perfectly moderates neutrons, but with the absorption of neutrons by hydrogen nucleus, secondary gamma rays with high energy are produced. These gamma rays highly penetrate matter, and therefore they can increase requirements on the thickness of the water shield. Adding a boric acid can help with this problem (neutron absorption on boron nuclei without strong gamma emission) but results in another problem with corrosion of construction materials.
Concrete as a neutron shield
The most commonly used neutron shielding in many nuclear science and engineering sectors is the shield of concrete. Concrete is also hydrogen-containing material, but unlike water, concrete has a higher density (suitable for secondary gamma shielding) and does not need any maintenance. Because concrete is a mixture of several different materials, its composition is not constant. So when referring to concrete as a neutron shielding material, the material used in its composition should be told correctly. Generally, concretes are divided into “ordinary “ concrete and “heavy” concrete. Heavy concrete uses heavy natural aggregates such as barites (barium sulfate) or magnetite or manufactured aggregates such as iron, steel balls, steel punch, or other additives. As a result of these additives, heavy concrete has a higher density than ordinary concrete (~2300 kg/m3). Very heavy concrete can achieve density up to 5,900 kg/m3 with iron additives or up to 8900 kg/m3 with lead additives. Heavy concrete provides very effective protection against neutrons.