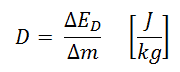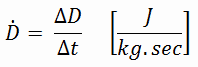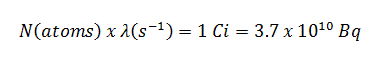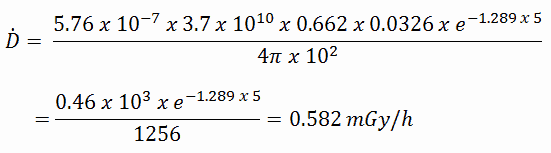Absorbed dose is defined as the amount of energy deposited by ionizing radiation in a substance. The absorbed dose is given the symbol D. The absorbed dose is usually measured in a unit called the gray (Gy), derived from the SI system. The non-SI unit rad is sometimes also used, predominantly in the USA.
- Gray. A dose of one gray is equivalent to a unit of energy (joule) deposited in a kilogram of a substance.
- RAD. A dose of one rad is equivalent to depositing one hundred ergs of energy in one gram of any material.
Why do we deal with a radiation dose? In previous chapters, we have discussed radioactivity and the intensity of a radioactive source usually measured in becquerels. But any radioactive source represents no biological risk as long as it is isolated from the environment. However, when people or another system (also non-biological) are exposed to radiation, energy is deposited in the material, and radiation dose is delivered.
It is therefore very important to distinguish between the radioactivity of a radioactive source and the radiation dose which may result from the source. Generally, the radiation dose depends on the following factors regarding the radioactive source:
- Activity. The activity of the source directly influences the radiation dose deposited in the material.
- Type of radiation. Each type of radiation interacts with matter differently. For example, charged particles with high energies can directly ionize atoms. On the other hand, electrically neutral particles interact indirectly but can also transfer some or all of their energies to the matter.
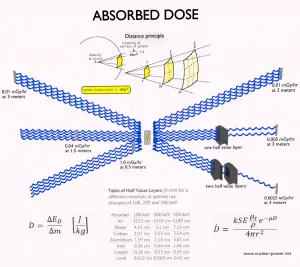
- Distance. The amount of radiation exposure depends on the distance from the radiation source. Similar to heat from a fire, if you are too close, the intensity of heat radiation is high, and you can get burned. If you are at the right distance, you can withstand it without any problems, and it is comfortable. If you are too far from a heat source, the insufficiency of heat can also hurt you. In a certain sense, this analogy can be applied to radiation also from radiation sources.
- Time. The amount of radiation exposure depends directly (linearly) on the time people spend near the radiation source.
- Shielding. Finally, the radiation dose also depends on the material between the source and the object. The shielding can be used if the source is too intensive and time or distance does not provide sufficient radiation protection.
The danger of ionizing radiation lies in the fact that the radiation is invisible and not directly detectable by human senses. People can neither see nor feel radiation, yet it deposits energy into the molecules of the body. The energy is transferred in small quantities for each interaction between the radiation and a molecule, and there are usually many such interactions.
In nuclear power plants, the central problem is to protect personals and the environment against gamma rays and neutrons because the ranges of charged particles (such as beta particles and alpha particles) in the matter are very short. On the other hand, we must deal with shielding all types of radiation because each nuclear reactor is a significant source of all types of ionizing radiation.
See also: Gamma Ray Attenuation
See also: Neutron Shielding
Gray – Unit of Absorbed Dose
A dose of one gray is equivalent to a unit of energy (joule) deposited in a kilogram of a substance. This unit was named in honor of Louis Harold Gray, who was one of the great pioneers in radiation biology. One gray is a large amount of absorbed dose. A person who has absorbed a whole-body dose of 1 Gy has absorbed one joule of energy in each kg of body tissue.
Absorbed doses measured in the industry (except nuclear medicine) often have usually lower doses than one gray, and the following multiples are often used:
1 mGy (milligray) = 1E-3 Gy
1 µGy (microgray) = 1E-6 Gy
Conversions from the SI units to other units are as follows:
- 1 Gy = 100 rad
- 1 mGy = 100 mrad
The gray and rad are physical units describing the incident radiation’s physical effect (i.e., the amount of energy deposited per kg). Still, it tells us nothing about the biological consequences of such energy deposition in living tissue.
Absorbed Dose Rate
The absorbed dose rate is the rate at which an absorbed dose is received and a measure of radiation dose intensity (or strength). The absorbed dose rate is therefore defined as:
In conventional units, it is measured in mrad/sec, rad/hr, mGy/sec or Gy/hr. Since the amount of radiation exposure depends directly (linearly) on the time people spend near the source of radiation, the absorbed dose is equal to the strength of the radiation field (dose rate) multiplied by the length of time spent in that field. The example above indicates a person could expect to receive a dose of 25 millirems by staying in a 50 millirems/hour field for thirty minutes.
Examples of Absorbed Doses in grays
We must note that radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us, and it is a part of our natural world that has been here since the birth of our planet. In the following points, we try to express enormous ranges of radiation exposure, which can be obtained from various sources.
- 0.05 µGy – Sleeping next to someone
- 0.09 µGy – Living within 30 miles of a nuclear power plant for a year
- 0.1 µGy – Eating one banana
- 0.3 µGy – Living within 50 miles of a coal power plant for a year
- 10 µGy – Average daily dose received from natural background
- 20 µGy – Chest X-ray
- 40 µGy – A 5-hour airplane flight
- 600 µGy – mammogram
- 1 000 µGy – Dose limit for individual members of the public, total effective dose per annum
- 3 650 µGy – Average yearly dose received from natural background
- 5 800 µGy – Chest CT scan
- 10 000 µGy – Average yearly dose received from a natural background in Ramsar, Iran
- 20 000 µGy – single full-body CT scan
- 175 000 µGy – Annual dose from natural radiation on a monazite beach near Guarapari, Brazil.
- 5 000 000 µGy – Dose that kills a human with a 50% risk within 30 days (LD50/30) if the dose is received over a very short duration.
As can be seen, low-level doses are common in everyday life. The previous examples can help illustrate relative magnitudes. From biological consequences, it is very important to distinguish between doses received over short and extended periods. An “acute dose” occurs over a short and finite period, while a “chronic dose” is a dose that continues for an extended period so that it is better described by a dose rate. High doses tend to kill cells, while low doses tend to damage or change them. Low doses spread out over long periods don’t cause an immediate problem to any body organ. The effects of low radiation doses occur at the cell level, and the results may not be observed for many years.
Calculation of Shielded Dose Rate
Assume the point isotropic source contains 1.0 Ci of 137Cs and has a half-life of 30.2 years. Note that the relationship between half-life and the amount of a radionuclide required to give an activity of one curie is shown below. This amount of material can be calculated using λ, which is the decay constant of certain nuclide:
About 94.6 percent decays by beta emission to a metastable nuclear isomer of barium: barium-137m. The main photon peak of Ba-137m is 662 keV. For this calculation, assume that all decays go through this channel.
Determine the primary photon dose rate, in gray per hour (Gy.h-1), at the outer surface of a 5 cm thick lead shield. The primary photon dose rate neglects all secondary particles. Assume that the effective distance of the source from the dose point is 10 cm. We shall also assume that the dose point is soft tissue and it can reasonably be simulated by water, and we use the mass-energy absorption coefficient for water.
See also: Gamma Ray Attenuation
See also: Shielding of Gamma Rays
Solution:
The primary photon dose rate is attenuated exponentially, and the dose rate from primary photons, taking account of the shield, is given by:
As can be seen, we do not account for the buildup of secondary radiation. If secondary particles are produced, or the primary radiation changes its energy or direction, the effective attenuation will be much less. This assumption generally underestimates the true dose rate, especially for thick shields and when the dose point is close to the shield surface, but this assumption simplifies all calculations. For this case, the true dose rate (with the buildup of secondary radiation) will be more than two times higher.
To calculate the absorbed dose rate, we have to use the formula:
- k = 5.76 x 10-7
- S = 3.7 x 1010 s-1
- E = 0.662 MeV
- μt/ρ = 0.0326 cm2/g (values are available at NIST)
- μ = 1.289 cm-1 (values are available at NIST)
- D = 5 cm
- r = 10 cm
Result:
The resulting absorbed dose rate in grays per hour is then:
If we want to account for the buildup of secondary radiation, then we have to include the buildup factor. The extended formula for the dose rate is then:
From Absorbed Dose to Equivalent Dose
As was written, each type of radiation interacts with matter differently. For example, charged particles with high energies can directly ionize atoms. On the other hand, electrically neutral particles interact indirectly but can also transfer some or all of their energies to the matter. It would certainly simplify matters if the biological effects of radiation were directly proportional to the absorbed dose. Unfortunately, biological effects also depend on how the absorbed dose is distributed along the radiation path. Studies have shown that alpha and neutron radiation cause greater biological damage for a given energy deposition per kg of tissue than gamma radiation. It was discovered biological effects of any radiation increase with the linear energy transfer (LET). In short, the biological damage from high-LET radiation (alpha particles, protons, or neutrons) is much greater than that from low-LET radiation (gamma rays). This is because the living tissue can more easily repair damage from radiation spread over a large area than that concentrated in a small area. Because more biological damage is caused for the same physical dose (i.e., the same energy deposited per unit mass of tissue), one gray of alpha or neutron radiation is more harmful than one gray of gamma radiation. The fact that radiations of different types (and energies) give different biological effects for the same absorbed dose is described in terms of factors known as the relative biological effectiveness (RBE) and the radiation weighting factor (wR).
——–