Hardening of Metals
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, abrasion, indentation, or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. To improve the hardness of the pure metal, we can use different ways, which include:
- Hall-Petch Method
- Solid Solution Hardening (alloying)
- Work Hardening (Cold Working)
- Precipitation Hardening
- Transformation hardening
- Dispersion Hardening
- Surface Hardening
Precipitation Hardening – Age Hardening
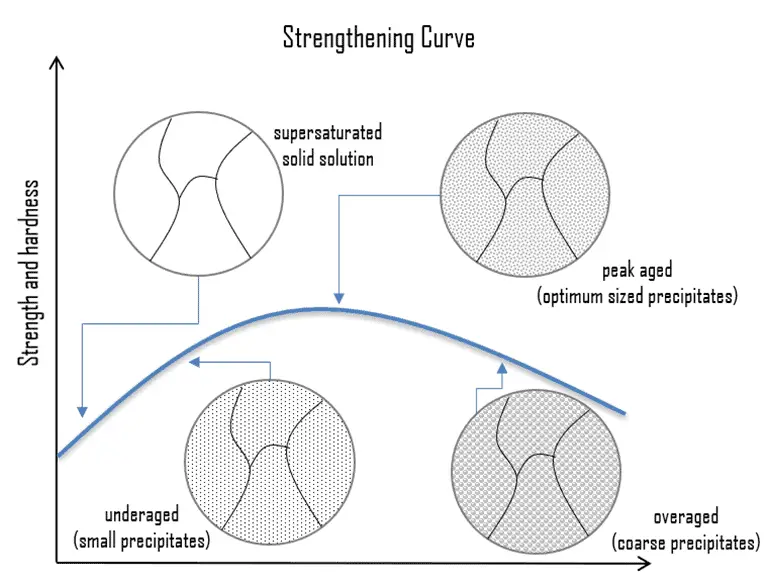 Precipitation hardening, called age hardening or particle hardening, is a heat treatment technique based on the formation of extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second-phase particles present further type of obstacles for dislocation movement, though the particles are not necessarily single atoms. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles that hinder the dislocation motion are either the strain field around the second phase particles, the second phase particles themselves, or both. Precipitation hardening increases the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, steel, and stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.
Precipitation hardening, called age hardening or particle hardening, is a heat treatment technique based on the formation of extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second-phase particles present further type of obstacles for dislocation movement, though the particles are not necessarily single atoms. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles that hinder the dislocation motion are either the strain field around the second phase particles, the second phase particles themselves, or both. Precipitation hardening increases the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, steel, and stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.
Nickel-based superalloys include solid-solution-strengthened alloys and age-hardenable alloys. Age-hardenable alloys are austenitic (fcc) matrix dispersed with coherent precipitation of a Ni3(Al, Ti) intermetallic with an fcc structure. Ni-based superalloys are alloys with nickel as the primary alloying element and are preferred as blade material in the previously discussed applications, rather than Co- or Fe-based superalloys. Ni-based superalloys are significant for their high strength, creep, and corrosion resistance at high temperatures. It is common to cast turbine blades in directionally solidified or single-crystal forms.
In the case of aluminium alloys, precipitation strengthening can increase the yield strength of aluminium from about five times up to about fifteen times that of unalloyed aluminium. Especially 2xxx series, alloyed with copper, can be precipitation hardened to strengths comparable to steel. In terms of age hardening, solution annealed aluminum-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding it at that temperature for a specified time. This process is called artificial aging. Typically, aluminum 6061 alloy is heat treated at 533°C (990°F) for a sufficient period, followed by quenching in water. The precipitation hardening process can be performed at 160°C (320°F) for 18 h, followed by air cooling. This process is repeated at 177°C (350°F) for 8 h, followed by cooling in the air.
In the case of copper beryllium, the high strength of this alloy is also attained by precipitation hardening. The precipitation hardening results from the precipitation of a beryllium-containing phase from a supersaturated solid solution of mostly pure copper. Copper beryllium is the hardest and strongest copper alloy (UTS up to 1,400 MPa) in fully heat treated, and cold worked conditions. It combines high strength with non-magnetic and non-sparking qualities. It is similar in mechanical properties to many high-strength alloy steels, but, compared to steels, it has better corrosion resistance.
17-4PH Stainless Steel
For example, precipitation-hardened stainless steel 17-4 PH (AISI 630) has an initial microstructure of austenite or martensite. Austenitic grades are converted to martensitic grades through heat treatment (e.g., heat treatment at about 1040 °C followed by quenching) before precipitation hardening. Subsequent aging treatment at about 475 °C precipitates Nb and Cu-rich phases, increasing the strength to above 1000 MPa yield strength. In all heat treatments performed, the predominant microstructure is lath martensite. Unlike austenitic alloys, heat treatment strengthens PH steel to levels higher than martensitic alloys. Precipitation-hardening stainless steels are designated by the AISI 600-series. Of all the available stainless grades, they generally offer the greatest combination of high strength, excellent toughness, and corrosion resistance. They are as corrosion-resistant as austenitic grades. Common uses are in the aerospace and some other high-technology industries.
Example – Aluminium Alloys – 6061 Alloy
 In general, 6000 series aluminium alloys are alloyed with magnesium and silicon. Alloy 6061 is one of the most widely used alloys in the 6000 Series. It has good mechanical properties, and it is easy to machine. It is weldable and can be precipitation hardened, but not to the high strengths that 2000 and 7000 can reach. It has very good corrosion resistance and weldability, although reduced strength in the weld zone. The mechanical properties of 6061 depend greatly on the material’s temper or heat treatment. Compared to the 2024 alloy, 6061 is more easily worked and remains resistant to corrosion even when the surface is abraded.
In general, 6000 series aluminium alloys are alloyed with magnesium and silicon. Alloy 6061 is one of the most widely used alloys in the 6000 Series. It has good mechanical properties, and it is easy to machine. It is weldable and can be precipitation hardened, but not to the high strengths that 2000 and 7000 can reach. It has very good corrosion resistance and weldability, although reduced strength in the weld zone. The mechanical properties of 6061 depend greatly on the material’s temper or heat treatment. Compared to the 2024 alloy, 6061 is more easily worked and remains resistant to corrosion even when the surface is abraded.