 Metals can be heat treated to alter the properties of strength, ductility, toughness, hardness, or resistance to corrosion. Several phenomena occur in metals and alloys at elevated temperatures. For example, recrystallization and the decomposition of austenite. These are effective in altering the mechanical characteristics when appropriate heat treatments or thermal processes are used. The use of heat treatments on commercial alloys is an exceedingly common practice. Common heat treatment processes include annealing, precipitation hardening, quenching, and tempering.
Metals can be heat treated to alter the properties of strength, ductility, toughness, hardness, or resistance to corrosion. Several phenomena occur in metals and alloys at elevated temperatures. For example, recrystallization and the decomposition of austenite. These are effective in altering the mechanical characteristics when appropriate heat treatments or thermal processes are used. The use of heat treatments on commercial alloys is an exceedingly common practice. Common heat treatment processes include annealing, precipitation hardening, quenching, and tempering.
Thermal Annealing
The term thermal annealing refers to a heat treatment in which a material is exposed to an elevated temperature for an extended period and then slowly cooled. This process alters a material’s physical and sometimes chemical properties to increase its ductility and reduce its hardness, making it more workable. In this process, atoms migrate in the crystal lattice, and the number of dislocations decreases, leading to a change in ductility and hardness. Metal gets rid of stresses and makes the grain structure large and soft-edged so that when the metal is hit or stressed it dents or perhaps bends rather than breaking. Typically, annealing is carried out to relieve stresses, increase softness, ductility, and toughness, and/or produce a specific microstructure.
Generally, in plain carbon steels, annealing produces a ferrite-pearlite micro-structure. Steels may be annealed to facilitate cold working or machining, to improve mechanical or electrical properties, or to promote dimensional stability. The most common structural steels produced have a mixed ferrite-pearlite microstructure. Their applications include beams for bridges and high-rise buildings, plates for ships, and reinforcing bars for roadways. These steels are relatively inexpensive and are produced in large tonnages.
Any annealing cycle consists of three stages:
- heating to the desired temperature,
- holding or “soaking” at that temperature,
- cooling, usually to room temperature.
Time and annealing temperature are important parameters in these procedures. Especially the target temperature defines the annealing thermal cycle.
Annealing Thermal Cycles
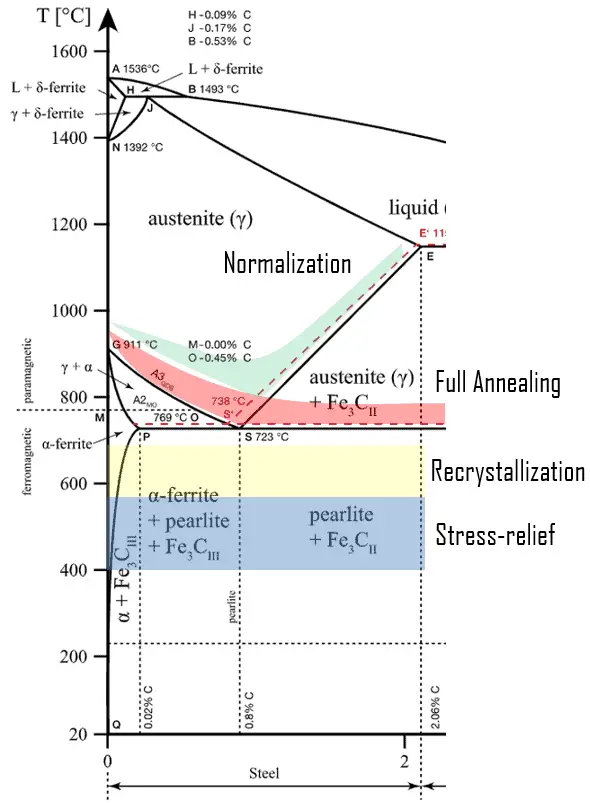 In practice, specific thermal cycles of an almost infinite variety are used to achieve the various goals of annealing. These cycles fall into several broad categories that can be classified according to the temperature at which the steel is heated and the cooling method used.
In practice, specific thermal cycles of an almost infinite variety are used to achieve the various goals of annealing. These cycles fall into several broad categories that can be classified according to the temperature at which the steel is heated and the cooling method used.
- Process Annealing. Process annealing is a specific heat treatment that restores some of the ductility to a product being cold-worked so it can be cold-worked further without breaking. It is commonly used during fabrication procedures that require extensive plastic deformation to allow a continuation of deformation without fracture or excessive energy consumption. The temperature range for process annealing ranges from 260 °C to 760 °C, depending on the alloy in question. This process is mainly suited for low-carbon steel carried out on cold-rolled steel like wire-drawn steel, centrifugally cast ductile iron pipe, etc.
- Stress-relief Annealing. Stress-relief annealing is used to relieve stresses from cold working. In contrast to process annealing, this heat treatment is usually performed after the product has been made. Care must ensure uniform cooling, particularly when a component is composed of variable section sizes. If the rate of cooling is not constant and uniform, new residual stresses can result in equal to or greater than those that the heat-treating process intended to relieve. The annealing temperature is typically relatively low, so effects resulting from cold working and other heat treatments are not affected. Stress-relief heat treating can reduce distortion and high stresses from welding that can affect service performance.
- Recrystallization Annealing. Recrystallization annealing of cold-worked steels (carbon content up to 0.5%) can produce a new grain structure without inducing a phase change. Metal is heated to a temperature at which the hardening caused by the previous cold-working is removed. During recrystallization, the internal bonds between the atoms change, and the crystal lattice does not change. Annealing temperatures are between 550-700 ° C, and the endurance is about 1 hour or more, cooling in air. It is used as inter-operational annealing in cold forming, especially for low-carbon parts.
- Full Annealing. Full annealing produces a microstructure that is softer and more amenable to other processing such as forming or machining. The temperatures for full annealing are typically 50 °C above the upper critical temperature (A3) for hypoeutectic steels and the lower critical temperature (A1) for hypereutectoid steels. It is referred to as full annealing because it achieves full austenitization of hypoeutectoid steels. The alloy is then furnace cooled. That means the heat-treating furnace is turned off, and both furnace and steel cool to room temperature simultaneously, which takes several hours. The cooling rate of the steel has to be sufficiently slow so as not to let the austenite transform into bainite or martensite but rather have it completely transform to pearlite and ferrite or cementite. A full anneal typically results in the second most ductile state a metal can assume for metal alloy. The metal attains relatively low levels of hardness, yield strength, and ultimate strength with high plasticity and toughness. Full annealing is often used in low- and medium-carbon steels that will be machined or experience extensive plastic deformation during forming. Stainless and high-alloy steels may be austenitized (fully annealed) and quenched to minimize the presence of grain boundary carbides or to improve the ferrite distribution.
- Normalizing. Normalization is an annealing process applied to ferrous alloys to refine grain size, make its structure more uniform, make it more responsive to hardening, and improve machinability. Normalizing is performed on steels plastically deformed by, for example, a rolling operation. These cold worked steels consist of grains of pearlite, which are irregularly shaped and relatively large and vary substantially in size. Normalizing is an austenitizing heating cycle followed by cooling in still or slightly agitated air. Typically, the temperatures for normalizing are approximately 55 °C above the upper critical line. The normalization temperature is higher than the temperature for full annealing. On the other hand, the cooling is more intense. Normalizing improves the machinability of a component and provides dimensional stability if subjected to further heat treatment processes. The main difference between annealing and normalizing is that annealing allows the material to cool at a controlled rate in a furnace. Normalizing allows the material to cool by placing it in a room-temperature environment and exposing it to the air in that environment.
Reactor Pressure Vessel Annealing
 The body of the reactor vessel is constructed of high-quality low-alloy carbon steel. To minimize corrosion, all surfaces that come into contact with reactor coolant are clad with a minimum of about 3 to 10 mm of austenitic stainless steel.
The body of the reactor vessel is constructed of high-quality low-alloy carbon steel. To minimize corrosion, all surfaces that come into contact with reactor coolant are clad with a minimum of about 3 to 10 mm of austenitic stainless steel.
During the operation of a nuclear power plant, the material of the reactor pressure vessel and the material of other reactor internals are exposed to neutron radiation (especially to fast neutrons >0.5MeV), which results in localized embrittlement of the steel and welds in the area of the reactor core. This phenomenon, known as irradiation embrittlement, results in:
- Steadily increase in DBTT. It is not likely that the DBTT will approach the normal operating temperature of the steel. However, there is a possibility that when the reactor is shut down or during an abnormal cooldown, the temperature may fall below the DBTT value. At the same time, the internal pressure is still high.
- Drop in the upper shelf fracture energy. Radiation effects are also manifested by a drop in the upper shelf fracture energy and a decrease in fracture toughness.
Plant operators must monitor all these effects. Therefore nuclear regulators require that a reactor vessel material surveillance program be conducted in water-cooled power reactors.
Once a material of RPV is degraded by radiation embrittlement (e.g., a significant increase in Charpy ductile‐brittle transition temperature or reduction of fracture toughness), thermal annealing of the RPV is the only way to recover the RPV material toughness properties.
According to 10 CFR 50.66 – Requirements for thermal annealing of the reactor pressure vessel:
“For those light water nuclear power reactors where neutron radiation has reduced the fracture toughness of the reactor vessel materials, thermal annealing may be applied to the reactor vessel to recover the material’s fracture toughness. “
Thermal annealing (“dry “method) of the reactor pressure vessel is a method by which the pressure vessel (with all reactor internals removed) is heated up to some temperature (usually between 420 – 460 °C) by use of an external heat source (electrical heaters, hot air), held for a given period (e.g., 100 – 200 hours) and then slowly cooled. The annealing equipment is usually a ring‐shaped furnace with heating elements on its external surface. The power output of installed heaters may reach up to 1 MWe. It was shown that the upper shelf recovered 100 % after the specially fabricated materials after 24 hours of annealing and more rapidly than transition temperature. Annealing for 168 hours recovered 90 % of the transition temperature shift.
Wet Annealing
There is also a possibility of the so‐called “wet” annealing method being applied in the USA and Belgium. The annealing at that temperature of ~340 °C was reached without external heating but by increasing the coolant temperature achieved by the energy of the circulating pumps of the primary circuit. This type of annealing provides only partial recovery for the material due to the limitation in maximum temperature.
Special Reference: Annealing and re-embrittlement of reactor pressure vessel materials. AMES report N.19; ISSN 1018-5593. European Communities, 2008.
Other Processes
- Annealing. The term annealing refers to a heat treatment in which a material is exposed to an elevated temperature for an extended period and then slowly cooled. In this process, metal gets rid of stresses and makes the grain structure large and soft-edged so that when the metal is hit or stressed it dents or perhaps bends rather than breaking; it is also easier to sand, grind, or cut annealed metal.
- Quenching. The term quenching refers to a heat treatment in which a material is rapidly cooled in water, oil, or air to obtain certain material properties, especially hardness. In metallurgy, quenching is commonly used to harden steel by introducing martensite. There is a balance between hardness and toughness in any steel; the harder the steel, the less tough or impact-resistant it is, and the more impact-resistant it is, the less hard it is.
- Tempering. The term tempering refers to a heat treatment used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening to reduce some of the excess hardness. It is done by heating the metal to some temperature below the critical point for a certain period, then allowing it to cool in still air. Tempering makes the metal less hard while enabling it to sustain impacts without breaking. Tempering will cause the dissolved alloying elements to precipitate, or in the case of quenched steels, improve impact strength and ductile properties.
- Aging. Age hardening also called precipitation hardening, or particle hardening is a heat treatment technique based on the formation of extremely small, uniformly dispersed particles of a second phase within the original phase matrix to enhance The strength and hardness of some metal alloys. Precipitation hardening increases the yield strength of malleable materials, including most structural alloys of aluminum, magnesium, nickel, titanium, steel, and stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.