 In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. A contracting substance may consist of another surface, a fluid, or hard, abrasive particles contained in some form of fluid or suspension, such as a lubricant. As is with friction, the presence of wear can be either good or bad. Productive, controlled wear can be found in processes like machining, cutting, grinding, and polishing. However, in most technological applications, the occurrence of wear is highly undesirable, and it is an enormously expensive problem since it leads to the deterioration or failure of components. In terms of safety, it is often not as serious (or as sudden) as a fracture, and this is because the wear is usually anticipated.
In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. A contracting substance may consist of another surface, a fluid, or hard, abrasive particles contained in some form of fluid or suspension, such as a lubricant. As is with friction, the presence of wear can be either good or bad. Productive, controlled wear can be found in processes like machining, cutting, grinding, and polishing. However, in most technological applications, the occurrence of wear is highly undesirable, and it is an enormously expensive problem since it leads to the deterioration or failure of components. In terms of safety, it is often not as serious (or as sudden) as a fracture, and this is because the wear is usually anticipated.
Certain material characteristics such as hardness, carbide type, and volume percent can have a decided impact on the wear resistance of a material in a given application. Wear, like corrosion, has multiple types and subtypes that are predictable to some extent and are rather difficult to test and evaluate in the lab or service reliably.
Tribology
Tribology is the interdisciplinary science of interacting surfaces in relative motion. Tribology includes the study and application of the principles of friction, lubrication, and wear under applied load. Efficient operation and robust performance of these machine components depend highly on tribology. Controlling friction helps to reduce energy loss and improve the efficiency and lifetime of machines. Wear of material from a surface can lead to severe damage and failure of the component and/or the machine. The most common approach to reduce friction and minimize wear and catastrophic failure of machine components or the machine itself is a proper selection of materials and lubricants. Most machine components use lubricants to reduce friction and prevent wear.
Surface Hardness and Wear Resistance
Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness. If the hardness of the material is higher than that of the abrasive material, less wear rate will occur.
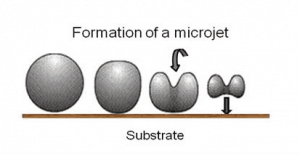
Case hardening or surface hardening is the process in which the hardness of an object’s surface (case) is enhanced while the inner core of the object remains elastic and rigid. After this process enhances surface hardness, wear resistance, and fatigue life. This is accomplished by several processes, such as a carburizing or nitriding process by which a component is exposed to a carbonaceous or nitrogenous atmosphere at elevated temperatures. As was written, two main material characteristics are influenced:
- Hardness and wear resistance is significantly enhanced. In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, abrasion, an indentation, or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
- Toughness is not negatively influenced, and toughness is the ability of a material to absorb energy and plastically deform without fracturing. One definition of toughness (for high-strain rate, fracture toughness) is that it is a property that is indicative of a material’s resistance to fracture when a crack (or other stress-concentrating defects) is present.
The case-hardening process involves infusing additional carbon or nitrogen into the surface layer of iron or steel with low carbon content, which has poor hardenability. Case hardening is useful in parts such as a cam or ring gear that must have a very hard surface to resist wear and a tough interior to resist the impact that occurs during operation. Further, the surface hardening of steel has an advantage over hardening (that is, hardening the metal uniformly throughout the piece) because less expensive low-carbon and medium-carbon steels can be surface hardened without the problems of distortion and cracking associated with the through hardening of thick sections. An atomic diffusion from the gaseous phase introduces a carbon- or nitrogen-rich outer surface layer (or case). The case is normally 1 mm deep and harder than the material’s inner core.
Wear Coefficient
Wear can be quantified (correlated) using wear rate, defined as the mass or volume of material removed per unit sliding distance. It is usually expressed in terms of the dimensionless wear coefficient (K) or as a specific wear rate (wear volume per unit applied normal load per unit sliding distance) in (mm3*Nm-1).
The most commonly used wear equation for the dry rolling–sliding condition is Archards wear equation. The wear volume (V), for unit sliding distance (S), is equal to the non-dimensional wear coefficient (K) multiplied by the applied load (Fn) divided by the hardness of the worn material.
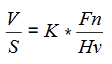
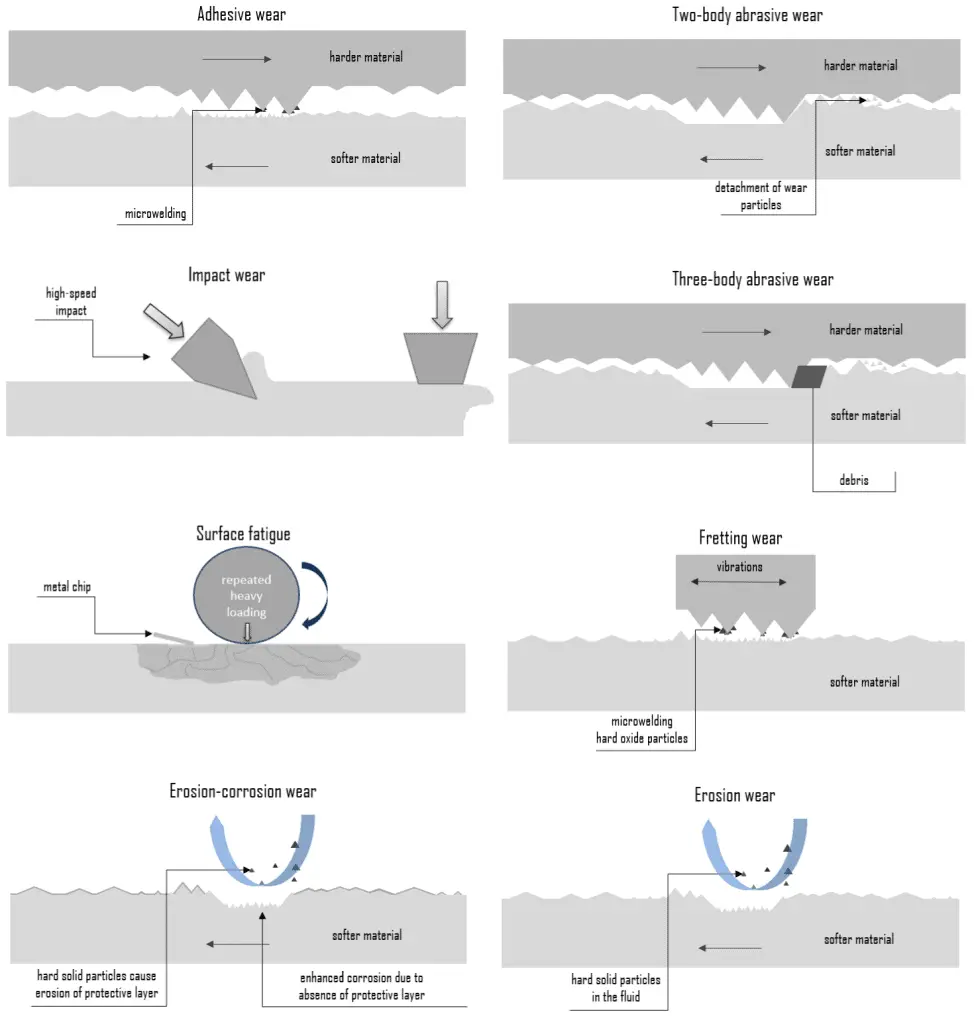
Wear Types
Wear is commonly classified according to so-called wear types, which occur in isolation or complex interaction. Wear mechanisms and/or sub-mechanisms frequently overlap and occur synergistically, producing a greater rate of wear than the sum of the individual wear mechanisms. The most common wear types are:
Other, less common types of wear are:
Abrasive Wear
Abrasive wear is defined as material loss due to hard particles or protuberances forced against and moving along a solid surface. It occurs when a hard rough surface slides across a softer surface. This mechanism is sometimes referred to as grinding wear. The harder material may be one of the rubbing surfaces or hard particles that have found their way between the mating surfaces. These may be ‘foreign’ particles or particles resulting from adhesive or delamination wear. Abrasion mainly involves microscale cutting and plowing processes. How an asperity slides over a surface determines the nature and intensity of abrasive wear. There are two basic modes of abrasive wear:
- Two-body abrasive wear. Two-body wear occurs when the grits or hard particles remove material from the opposite surface. The common analogy is that material is removed or displaced by a cutting or plowing operation.
- Three-body abrasive wear. Three-body wear occurs when the particles are not constrained and are free to roll and slide down a surface. The contact environment determines whether the wear is classified as open or closed. An open contact environment occurs when the surfaces are sufficiently displaced to be independent of one another.
There are many different strategies for mitigating abrasive wear, but the general rule for materials selection is that the harder, the better. Materials containing a relatively large percentage of hard, wear-resistant alloy carbides, such as selected tools and high-speed steels.
Adhesive Wear
Adhesive wear originated through bonding asperities or microscopic high points (surface roughness) between two sliding materials. When a peak from one surface comes into contact with a peak from the other surface, instantaneous micro-welding may take place due to the heat generated by the resulting friction. This results in detachment or material transfer from one surface to the other. For adhesive wear to occur, the surfaces must be in intimate contact. This may cause unwanted displacement and attachment of wear debris and material compounds from one surface to another. Adhesive wear can lead to an increase in roughness and the creation of protrusions (i.e., lumps) above the original surface. Surfaces held apart by lubricating films, oxide films, etc., reduce the tendency for adhesion. In some engineering applications, surfaces slide in the air without lubricant, and the resulting wear is termed dry sliding.
Adhesive wear depends on the materials involved, the degree of lubrication provided, and the environment. Adequate lubrication allows smooth, continuous operation of machine elements, reduces wear rate, and prevents excessive stresses or seizures at bearings. When lubrication breaks down, components can rub destructively against each other, causing heat, local welding, destructive damage, and failure. For instance, austenitic stainless steels (e.g., AISI 304) sliding against themselves are very likely to transfer material and gall, resulting in severe surface damage. Other materials prone to adhesive wear include titanium, nickel, and zirconium. On the other hand, aluminum bronze has found increasing recognition for various applications requiring resistance to mechanical wear. Its wear resistance is based on the transfer from the softer metal (aluminum bronze) to the harder metal (steel), forming a thin layer of softer metal on the harder metal.
For example, the main function of motor oil is to reduce friction and wear on moving parts (to reduce adhesive wear) and to clean the engine from sludge. At the same time, a filter is designed to remove contaminants and abrasive particles from engine oil.
Erosion Wear
Erosion wear is a process of progressive material removal from a target surface due to repeated impacts of solid particles. The particles suspended in the flow of solid-liquid mixture erode the wetted passes limiting the service life of the equipment used for the slurry transportation system. Each particle cuts or fractures a tiny amount of material (known as wear chips) from the surface. If this is repeated over a long period, a significant amount of material loss can result.
Erosive wear is common in pumps, impellers, fans, steam lines, and nozzles inside sharp bends in tubes and pipes. Therefore, it is a widely encountered mechanism in industry and power engineering. Due to the nature of the conveying process, piping systems are prone to wear when abrasive particles have to be transported.
Erosion wear is caused by the kinetic energy transferred to the target surface by impinging solid particles. The rate of erosive wear is dependent upon many factors. The material characteristics of the particles, such as their shape, hardness, impact velocity, and impingement angle, are primary factors, along with the properties of the surface being eroded. A material loss of target material is higher for the higher kinetic energy of impinging particles. So impact velocity largely affects the erosion wear of target material. The impingement angle is one of the most important factors and is widely recognized in the literature. Sharp curves or bends tend to produce more erosion than gentle curves.
Erosion wear can be classified into three categories:
- Solid particle erosion. Solid particle erosion is the loss of material volume from a target material due to continuous impingement of solid particles in the flowing fluid.
- Liquid impact erosion. The continuous striking of liquid jet on the material surface causes liquid impact erosion.
- Cavitations erosion. The vapor or gas in a liquid forms cavities or bubbles that cause wear.
In general, erosive wear resistance may be improved by increasing the hardness of the surface, by proper materials, and by proper design of the product. Some specific steps that can be taken to change flow conditions include: reducing fluid velocity, eliminating turbulence at misalignments, and avoiding sharp bends.
Erosion – Corrosion
Erosion can also occur in combination with other forms of degradation, such as corrosion. This is referred to as erosion-corrosion. Erosion corrosion is a material degradation process due to the combined effect of corrosion and wear. Nearly all flowing or turbulent corrosive media can cause erosion corrosion. The mechanism can be described as follows:
- mechanical erosion of the material, or protective (or passive) oxide layer on its surface,
- enhanced corrosion of the material if the corrosion rate of the material depends on the thickness of the oxide layer.
Wear is a mechanical material degradation process occurring on rubbing or impacting surfaces, while corrosion involves chemical or electrochemical reactions of the material. Corrosion may accelerate wear, and wear may accelerate corrosion.
Fretting Wear
Fretting wear is a special wear process at the contact area between two materials under load and is subject to a minute relative motion by vibration or some other force. Fretting wear is similar to adhesive wear in that micro-welding occurs on mating surfaces. In adhesive wear, however, facing metals slide across each other, while in fretting wear, metal-to-metal interfaces are essentially stationary. The amplitude of the relative sliding motion is often in the order from micrometers to millimeters. Because fretting wear is essentially a stationary phenomenon, debris is retained at or near the locations where it was formed originally. This debris usually consists of oxides of the metals in contact. Because the oxidized debris is usually much harder than the surfaces from which it came, it often acts as an abrasive agent that increases the rate of fretting. Fretting wear typically occurs in bearings, although most bearings have their surfaces hardened to resist the problem.
Fretting mitigation is based on the following measures:
- Reduce or eliminate vibration. The fundamental way to prevent fretting is to design for no relative motion of the surfaces at the contact
- Use of an elastomeric material to prevent metal-to-metal contact.
- Lubrication. The problem is that liquid lubricant cannot flow through the interface because the joint is stationary.
Debris Fretting – Grid-to-rod Fretting
In nuclear power plants, fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is made of corrosion-resistant material with a low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. In the early dates of PWR and BWR operations, fretting was one of the main failure mechanisms of this fuel cladding. It typically has two variants.
- Debris fretting. Debris fretting can be caused by any debris (foreign material – usually metallic) that can enter the fuel bundle and potentially become lodged between the spacer grid and a fuel rod. Fretting wear of fuel cladding can result in cladding penetration.
- Grid-to-rod fretting. Grid-to-rod fretting arises from the vibration of the fuel element generated by the high coolant velocity through the spacing grid. Spacing grids are welded onto the guide tubes and ensured using springs and dimples, fuel rod support, and spacing. High coolant velocity can cause the rod to rub against the part of the spacer grid that holds it. This type of cladding wear can be minimized by properly designing the spacing grid. Baffle-jetting is usually grouped under grid-to-rod fretting.
See also: IAEA, Review of fuel failures in water-cooled reactors. No. NF-T-2.1. ISBN 978–92–0–102610–1, Vienna, 2010.
Surface Fatigue – Fatigue Wear
In materials science, fatigue is the weakening of a material caused by cyclic loading that results in progressive, brittle, and localized structural damage. Surface fatigue, or fatigue wear, is the cracking and subsequent pitting of surfaces subjected to alternating stresses during rolling contact or the stresses from combined rolling and sliding. Fatigue wear is produced when the wear particles are detached by cyclic crack growth of microcracks on the surface. These microcracks are either superficial cracks or subsurface cracks. The repeated loading and unloading cycles to which the materials are exposed may induce the formation of subsurface or surface cracks, which eventually, after a critical number of cycles, will result in the breakup of the surface with the formation of large fragments, leaving large pits in the surface. Once a crack has been initiated, each loading cycle will grow the crack a small amount, even when repeated alternating or cyclic stresses are of an intensity considerably below the normal strength. The stresses could be due to vibration or thermal cycling. Subsurface and surface fatigues are observed during repeated rolling and sliding, respectively.
Corrosive and oxidation wear
Corrosive wear is a material degradation process due to the combined effect of corrosion and wear. It is defined as the wear process in which sliding occurs in a corrosive environment. In the absence of sliding, the products of the corrosion (e.g., oxides) would form a film typically less than a micrometer thick on the surfaces, which would tend to slow down or even eliminate the corrosion. Still, the sliding action wears the film away so that further corrosion can continue. Oxidation wear is one of the most common forms of corrosive wear because an oxygen-rich environment is a typical environment in which this wear process occurs. Corrosive wear requires both corrosion and rubbing. Chemical corrosion occurs in highly corrosive environments and high temperature and high humidity environments.
Erosion – Corrosion
Erosion can also occur in combination with other forms of degradation, such as corrosion. This is referred to as erosion-corrosion. Erosion corrosion is a material degradation process due to the combined effect of corrosion and wear. Nearly all flowing or turbulent corrosive media can cause erosion corrosion. The mechanism can be described as follows:
- mechanical erosion of the material, or protective (or passive) oxide layer on its surface,
- enhanced corrosion of the material if the corrosion rate of the material depends on the thickness of the oxide layer.
Wear is a mechanical material degradation process occurring on rubbing or impacting surfaces, while corrosion involves chemical or electrochemical reactions of the material. Corrosion may accelerate wear, and wear may accelerate corrosion.
Impact Wear
Impact wear is defined as the wear of a solid surface due to percussion, which is repetitive exposure to dynamic contact by another solid body. Impact wear is of the highest importance in mining and mineral processing. Mining and mineral processing demand wear-resistant machines and components because the energies and masses of interacting bodies are significant. For this purpose, materials with the highest wear resistance must be used. For example, tungsten carbide is used extensively in mining in top hammer rock drill bits, downhole hammers, roller-cutters, long wall plow chisels, long wall shearer picks, raise-boring reamers, and tunnel boring machines.
For metal impact pairs, the behavior of impact wear involves elastic and plastic deformation when impact loading or impact energy is high and/or fatigue is accompanied by wear debris release due to crack formation. In general, impact wear of metals depends on deformed layers’ formation, particularly when wear by fatigue or crack formation is predominant. In such cases, subsurface cracks extend parallel to the surface, similar to delamination wear. The sufficient hardness of the impacted component is necessary to prevent rapid wear or extrusion of material from the contact by plastic deformation. In most situations, this condition can be fulfilled by assuring an adequate hardness and then wear controlled by other material characteristics.
Cavitation wear
Cavitation wear is a process of progressive degradation of a material due to repeated nucleation, growth, and violent collapse of cavities in a liquid flowing near the material. Cavitation fatigue is a specific type of damage mechanism caused by repeated vibration and movement due to contact with flowing liquids, with water being the most common fluid. Cavitation is, in many cases, an undesirable occurrence. In centrifugal pumps, cavitation causes damage to components (erosion of the material), vibrations, noise, and a loss of efficiency.

https://commons.wikimedia.org/wiki/File:Turbine_Francis_Worn.JPG
Perhaps the most critical engineering problem caused by cavitation is the material damage that cavitation bubbles can cause when they collapse in the vicinity of a solid surface. Cavitation bubble collapse is a violent process that generates highly localized shock waves and microjets. They force energetic liquid into very small volumes, thereby creating spots of high temperature, and these intense disturbances generate highly localized, transient surface stresses to a solid surface. Signs of erosion will appear as pitting due to the water-hammering action of the collapsing vapor bubbles. It has been found that cavitation damage rates increase rapidly with the increase in the volume flow rate.
Softer materials can be damaged even by the short-term occurrence of cavitation, and individual pits can be observed after a single bubble collapse. Therefore harder materials are used for centrifugal pumps. But with the harder materials used in most applications, the cyclic stress due to repeated collapses can cause local surface fatigue failure. Thus cavitation damage to metals usually has the appearance of fatigue failure.
 When the cavitation bubbles collapse, they force energetic liquid into very small volumes, thereby creating spots of high temperature and emitting shock waves, the latter of which are a source of the noise. Although the collapse of a small cavity is a relatively low-energy event, highly localized collapses can erode metals, such as steel, over time. The pitting caused by the failure of cavities produces great wear on components and can dramatically shorten a propeller or pump’s lifetime.
When the cavitation bubbles collapse, they force energetic liquid into very small volumes, thereby creating spots of high temperature and emitting shock waves, the latter of which are a source of the noise. Although the collapse of a small cavity is a relatively low-energy event, highly localized collapses can erode metals, such as steel, over time. The pitting caused by the failure of cavities produces great wear on components and can dramatically shorten a propeller or pump’s lifetime.
Cavitation is usually accompanied also by:
- Noise. Collapsing cavities cause typical noise, and the level of noise that results from cavitation is a measure of the severity of the cavitation.
- Vibration. Pump vibrations due to cavitation are characteristically low-frequency vibrations, usually found in the 0 to 10 Hz range.
- Reduction in pump efficiency. Decreasing the pump’s efficiency is a more reliable sign of cavitation.
Prevention of Cavitation
Pits can vary from very small to very large, or even they can completely penetrate the thickness of metal. Damage to the structure can be catastrophic, and losses in functional efficiency can be substantial. Methods of dealing with this problem include:
- Increasing the hardness and strength of the metal. However, this may only delay the problem rather than prevent it.
- Increasing the stiffness of the part. This should reduce its amplitude of vibration, thereby increasing its natural vibration frequency. It may be possible to increase wall thickness or add stiffening ribs to change vibration characteristics.
- Increasing the smoothness of the surface. Cavities tend to cluster in certain low-pressure areas, and this dispersing of the cavities may be possible to eliminate surface peaks and valleys.
Diffusive wear
Diffusion or dissolution wear refers to the damage, erosion, or degradation of materials on a metal’s surface due to increased surface temperatures. When two materials are in contact, atoms from one material could diffuse into the other, causing diffusion or dissolution wear. Diffusive wear is primarily due to the heat of adhesion when two rough surfaces move across each other, typically when one metal is sliding across the other.
Typical Wear-resistant Materials
In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. Therefore, there is perfect wear-resistant material; in every case, it depends strongly on many variables (e.g., materials combination, contact pressure, environment, temperature). The hardness of the material is correlated to the wear resistance of the material. If the hardness of the material is less than that of the hardness of the abrasive material, then the wear rate is high. The hardness of material plays a major role in wear resistance. Some materials exhibit special wear characteristics:
- Ni3Al – Alloy. Nickel aluminide is an intermetallic alloy of nickel and aluminum with properties similar to ceramic and metal. Nickel aluminide is unique because it has very high thermal conductivity combined with high strength at high temperatures. These properties, combined with its high strength and low density, make it ideal for special applications like coating blades in gas turbines and jet engines. Composite materials with Ni3Al-based alloys as a matrix hardened by, e.g., TiC, ZrO2, WC, SiC, and graphene, are advanced materials. In 2005, the most abrasion-resistant material was reportedly created by embedding diamonds in a matrix of nickel aluminide.
- Tungsten Carbide. Impact wear is of the highest importance in mining and mineral processing. Mining and mineral processing demand wear-resistant machines and components because the energies and masses of interacting bodies are significant. For this purpose, materials with the highest wear resistance must be used. For example, tungsten carbide is used extensively in mining in top hammer rock drill bits, downhole hammers, roller-cutters, long wall plow chisels, long wall shearer picks, raise-boring reamers, and tunnel boring machines.
- Silicon Carbide. Silicon carbide is hard, synthetically produced silicon and carbon crystalline compound, and its chemical formula is SiC. Silicon carbide has a Mohs hardness rating of 9, approaching that of a diamond. In addition to hardness, silicon carbide crystals have fracture characteristics that make them extremely useful in grinding wheels. Its high thermal conductivity, high-temperature strength, low thermal expansion, and resistance to a chemical reaction make silicon carbide valuable in manufacturing high-temperature applications and other refractories.
- Coated Alloys. Case hardening by surface treatment can be further classified as diffusion or localized heating treatments. Diffusion methods introduce alloying elements that enter the surface by diffusion as solid-solution or hardenability agents that assist martensite formation during subsequent quenching. In this process, the concentration of alloying element is increased at the surface of a steel component. Diffusion methods include:
- Carburizing is a case hardening process in which the surface carbon concentration of a ferrous alloy (usually low-carbon steel) is increased by diffusion from the surrounding environment. Carburizing produces hard, highly wear-resistant surface (medium case depths) of product with an excellent capacity for contact load, good bending fatigue strength, and good resistance to seizure.
- Nitriding is a case hardening process in which the surface nitrogen concentration of a ferrous is increased by diffusion from the surrounding environment to create a case-hardened surface. Nitriding produces hard, highly wear-resistant surface (shallow case depths) of product with a fair capacity for contact load, good bending fatigue strength, and excellent resistance to seizure.
- Boriding, also called boronizing, is a thermochemical diffusion process similar to nitrocarburizing in which boron atoms diffuse into the substrate to produce hard and wear-resistant surface layers. The process requires a high treatment temperature (1073-1323 K) and long duration (1-12 h) and can be applied to a wide range of materials such as steels, cast iron, cermets, and non-ferrous alloys.
- Titanium-carbon and Titanium-nitride Hardening. Titanium nitride (an extremely hard ceramic material) or titanium carbide coatings can be used in the tools made of this kind of steel through a physical vapor deposition process to improve the performance and life span of the tool. TiN has a Vickers hardness of 1800–2100 and a metallic gold color.
 Case Hardened Steels. Case hardening based on martensitic transformation is usually performed to enhance the wear resistance of steels. Martensitic transformation hardening is one of the most common hardening methods, primarily used for steels (i.e., carbon steels and stainless steels).
Case Hardened Steels. Case hardening based on martensitic transformation is usually performed to enhance the wear resistance of steels. Martensitic transformation hardening is one of the most common hardening methods, primarily used for steels (i.e., carbon steels and stainless steels).- Flame hardening. Flame hardening is a surface hardening technique that uses a single torch with a specially designed head to provide a very rapid means of heating the metal, which is then cooled rapidly, generally using water. This creates a “case” of martensite on the surface, while the inner core of the object remains elastic and tough. It is a similar technique to induction hardening. The carbon content of 0.3–0.6 wt% C is needed for this type of hardening.
- Induction hardening. Induction hardening is a surface hardening technique that uses induction coils to provide a very rapid means of heating the metal, which is then cooled rapidly, generally using water. This creates a “case” of martensite on the surface. The carbon content of 0.3–0.6 wt% C is needed for this type of hardening.
- Laser hardening. Laser hardening is a surface hardening technique that uses a laser beam to provide a very rapid means of heating the metal, which is then cooled rapidly (generally by self-quenching). This creates a “case” of martensite on the surface, while the inner core of the object remains elastic and tough.
Some common materials:
-
Nibral Propeller (nickel aluminium bronze) Source: generalpropeller.com Ductile cast iron. Ductile iron, also known as nodular iron or spheroidal graphite iron, is very similar to gray iron in composition, but during solidification, the graphite nucleates as spherical particles (nodules) in ductile iron rather than as flakes. Typical applications for this material include valves, pump bodies, crankshafts, gears, and other automotive and machine components because of its good machinability, fatigue strength, and higher modulus of elasticity (compared to gray iron), and in heavy-duty gears because of its high yield strength and wear resistance.
- Aluminium Bronze. The aluminium bronzes are a family of copper-based alloys that combine mechanical and chemical properties unmatched by any other alloy series. They contain about 5 to 12% of aluminum. Aluminum bronze has found increasing recognition for various applications requiring resistance to mechanical wear. Its wear resistance is based on the transfer from the softer metal (aluminum bronze) to the harder metal (steel), forming a thin layer of softer metal on the harder metal.