Water is a transparent and nearly colorless substance composed of the chemical elements hydrogen and oxygen that are connected by covalent bonds. Water exists in gaseous (steam or water vapor), liquid, and solid states (ice), on Earth. It is one of the most plentiful and essential compounds, and it is the main constituent of Earth’s streams, lakes, oceans, and the fluids of most living organisms. It is vital for all known forms of life.
Water is a transparent and nearly colorless substance composed of the chemical elements hydrogen and oxygen that are connected by covalent bonds. Water exists in gaseous (steam or water vapor), liquid, and solid states (ice), on Earth. It is one of the most plentiful and essential compounds, and it is the main constituent of Earth’s streams, lakes, oceans, and the fluids of most living organisms. It is vital for all known forms of life.
Besides being essential to life, water is a remarkable substance with many surprising properties.
- It is the only chemical compound that occurs naturally in all three physical states – gaseous (steam or water vapor), liquid, and solid states (ice).
- It is the only inorganic liquid that occurs naturally on the Earth.
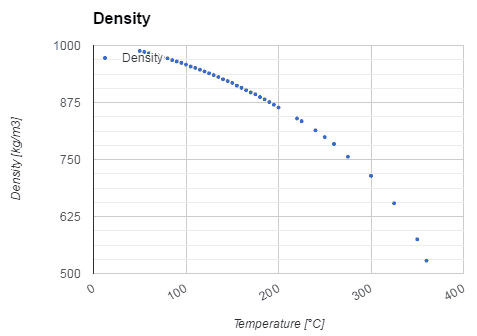
- Water also differs from most liquids in that it becomes less dense as it freezes. It has a maximum density of 3.98 °C (1000 kg/m3), whereas the density of ice is 917 kg/m3. It differs by about 9% and therefore ice floats on liquid water.
- Water has the highest specific heat of any common substance – 4.19 kJ/kg K.
- It has very high heat of vaporization, which makes it an effective coolant and medium in thermal power plants and other energy industry.
Uses of Water in Nuclear Engineering
Water as a reactor coolant
Water and steam are common fluids used for heat exchange in the primary circuit (from the surface of fuel rods to the coolant flow) and in the secondary circuit. It used due to its availability and high heat capacity, both for cooling and heating. It is especially effective to transport heat through vaporization and condensation of water because of its very large latent heat of vaporization.
A disadvantage is that water moderated reactors have to use the high-pressure primary circuit to keep water in the liquid state and achieve sufficient thermodynamic efficiency. Water and steam also react with metals commonly found in industries such as steel and copper, oxidized faster by untreated water and steam. In almost all thermal power stations (coal, gas, nuclear), water is used as the working fluid (used in a closed loop between boiler, steam turbine and condenser), and the coolant (used to exchange the waste heat to a water body or carry it away by evaporation in a cooling tower).
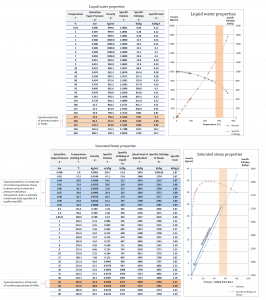
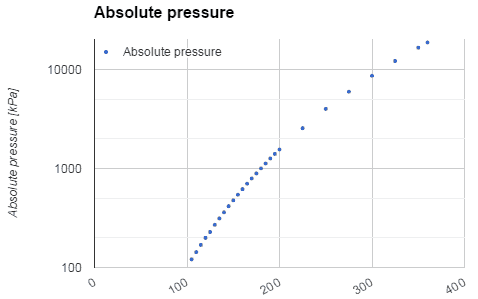
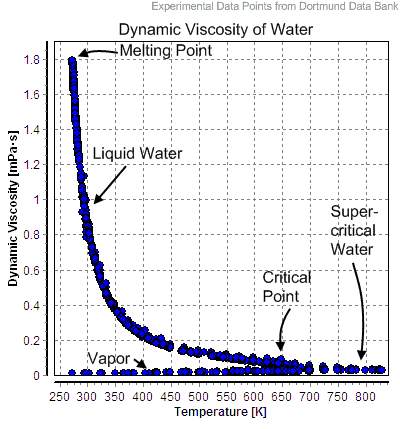
Water and steam are a common medium because their properties are very well known. Their properties are tabulated in so-called “Steam Tables”. In these tables, the basic and key properties, such as pressure, temperature, enthalpy, density, and specific heat, are tabulated along the vapor-liquid saturation curve as a function of both temperature and pressure. The properties are also tabulated for single-phase states (compressed water or superheated steam) on a grid of temperatures and pressures extending to 2000 ºC and 1000 MPa.
Further comprehensive authoritative data can be found at the NIST Webbook page on thermophysical properties of fluids.
See also: Steam Tables
Water as a moderator
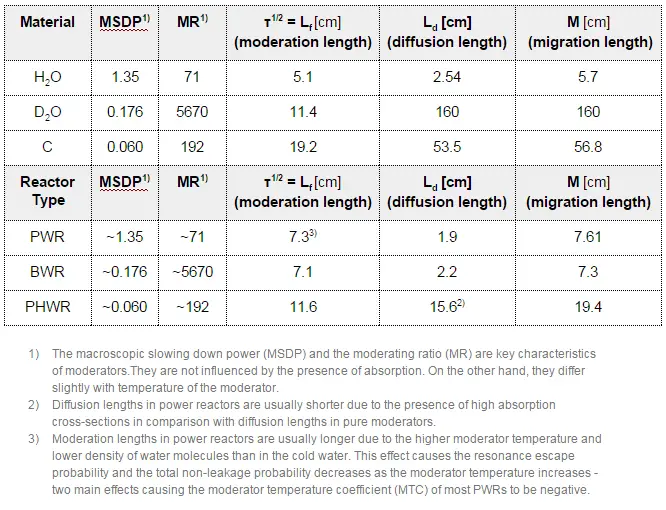
The neutron moderator, which is important in thermal reactors, is used to moderate, that is, to slow down neutrons from fission to thermal energies. Nuclei with low mass numbers are most effective for this purpose, so the moderator is always a low-mass-number material. Commonly used moderators include regular (light) water (roughly 75% of the world’s reactors), solid graphite (20% of reactors), and heavy water (5% of reactors).
In most nuclear reactors, water is both a coolant and a moderator. The moderation occurs especially on hydrogen nuclei. In the case of the hydrogen (A = 1) as the target nucleus, the incident neutron can be completely stopped – it has the highest average logarithmic energy decrement of all nuclei. On the other hand, hydrogen nuclei have a relatively higher absorption cross section. Therefore water is not the best moderator according to the moderating ratio.
Water as a neutron shielding
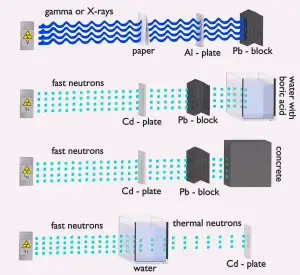
Water due to the high hydrogen content and the availability is efective and common neutron shielding. However, due to the low atomic number of hydrogen and oxygen, water is not an acceptable shield against gamma rays. On the other hand, in some cases, this disadvantage (low density) can be compensated by the high thickness of the water shield. In the case of neutrons, water perfectly moderates neutrons, but with the absorption of neutrons by hydrogen nuclei, secondary gamma rays with high energy are produced. These gamma rays highly penetrate matter, and therefore, they can increase requirements on the thickness of the water shield. Adding a boric acid can help with this problem (neutron absorbtion on boron nuclei without strong gamma emission) but results in other problems with corrosion of construction materials.
See also: Shielding of Neutrons
Water as a gamma radiation shielding
In short, effective shielding of gamma radiation is in most cases based on use of materials with two following material properties:
- high-density of material.
- high atomic number of material (high Z materials)
Although water is neither high density nor high Z material, it is commonly used as gamma shields. Water provides a radiation shielding of fuel assemblies in a spent fuel pool during storage or transports from and into the reactor core. Although water is a low-density material and low Z material, it is commonly used in nuclear power plants because these disadvantages can be compensated with increased thickness.
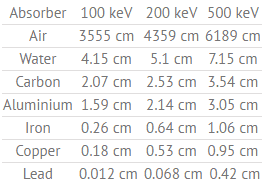
Half Value Layer of Water
The half value layer expresses the thickness of absorbing material needed for reduction of the incident radiation intensity by a factor of two.
Table of Half Value Layers (in cm) for a different materials at gamma ray energies of 100, 200 and 500 keV.