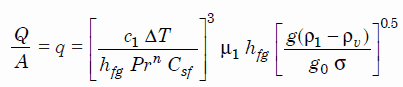The boiling regimes discussed above differ considerably in their character, and different correlations describe the heat transfer. This section reviews some of the more widely used correlations for nucleate boiling.
Nucleate Pool Boiling
Rohsenow correlation
The most widely used correlation for the rate of heat transfer in the nucleate pool boiling was proposed in 1952 by Rohsenow:
Rohsenow correlation
where
- q – nucleate pool boiling heat flux [W/m2]
- c1 — specific heat of liquid J/kg K
- ΔT — excess temperature °C or K
- hfg – enthalpy of vaporization, J/kg
- Pr — Prandtl number of liquid
- n — experimental constant equal to 1 for water and 1.7 for other fluids
- Csf — surface fluid factor, for example, water and nickel have a Csf of 0.006
- μ1 — dynamic viscosity of the liquid kg/m.s
- g – gravitational acceleration m/s2
- g0 — force conversion factor kgm/Ns2
- ρ1 — density of the liquid kg/m3
- ρv — density of vapor kg/m3
- σ — surface tension-liquid-vapor interface N/m
As can be seen, ΔT ∝ (q)⅓. This very important proportionality shows increasing ability of interface to transfer heat.