Natural Uranium
Natural uranium consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). Differences in the half-lives cause the abundance of isotopes in nature. All three naturally occurring isotopes of uranium (238U, 235U, and 234U) are unstable.
Uranium in the Environment
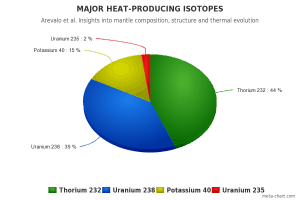
All three naturally occurring uranium isotopes (238U, 235U, and 234U) have a very long half-life (e.g.,, 4.47×109 years for 238U). Because of this very long half-life, uranium is weakly radioactive and contributes to low natural background radiation levels in the environment. These isotopes are alpha radioactive (emitting alpha particle), but they can also rarely spontaneously fission. All naturally occurring isotopes belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.54×109 years). Uranium has the second-highest atomic mass of these primordial nuclides, lighter only than plutonium. Moreover, the decay heat of uranium and its decay products (e.g.,, radon, radium, etc.) contributes to heating the Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.