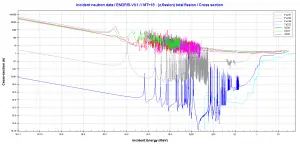
What is Plutonium
Plutonium is a transuranic chemical element with atomic number 94, which means there are 94 protons and 94 electrons in the atomic structure. The chemical symbol for plutonium is Pu. It is a manufactured isotope and is created from uranium in nuclear reactors. Therefore we can find this element in irradiated nuclear fuel or as fissile material in nuclear weapons. Scientists have found trace amounts of naturally occurring plutonium. Four isotopes (238Pu, 239Pu, 240Pu, and 244Pu) can be found in nature.
- Trace amounts of 239Pu originate in radiative capture of neutron on 238U. Free neutrons come from a spontaneous fission reaction of 238U.
- Trace amounts of 244Pu originate in its relatively long half-life of about 80 million years.
- The isotope 240Pu is in a decay chain of the isotope 244Pu. These nuclei are present in the unalterable proportions of the radioactive equilibrium between 244Pu and 240Pu.
- Extremely small amounts of 238Pu originate in extremely rare double negative beta decay of naturally-occurring isotope 238U.
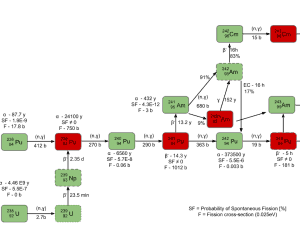
Plutonium is mostly produced in nuclear reactors. It is a product of the transmutation and subsequent nuclear decay of fertile isotope 238U. The transmutation and decay chain is shown below: Neutron capture may also be used to create fissile 239Pu from 238U, which is the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.Higher isotopes of plutonium (240Pu, 241Pu and 242Pu) are created by also by neutron radiative capture, but in this case an absorber must be the plutonium nucleus. For example, 240Pu which is the second most common isotope, is formed by radiative capture of a neutron by 239Pu.The transmutation and decay chain is shown below.
Neutron capture may also be used to create fissile 239Pu from 238U, which is the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.Higher isotopes of plutonium (240Pu, 241Pu and 242Pu) are created by also by neutron radiative capture, but in this case an absorber must be the plutonium nucleus. For example, 240Pu which is the second most common isotope, is formed by radiative capture of a neutron by 239Pu.The transmutation and decay chain is shown below.
 Neutron capture may also be used to create fissile 239Pu from 238U, which is the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.Higher isotopes of plutonium (240Pu, 241Pu and 242Pu) are created by also by neutron radiative capture, but in this case an absorber must be the plutonium nucleus. For example, 240Pu which is the second most common isotope, is formed by radiative capture of a neutron by 239Pu.The transmutation and decay chain is shown below.
Neutron capture may also be used to create fissile 239Pu from 238U, which is the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.Higher isotopes of plutonium (240Pu, 241Pu and 242Pu) are created by also by neutron radiative capture, but in this case an absorber must be the plutonium nucleus. For example, 240Pu which is the second most common isotope, is formed by radiative capture of a neutron by 239Pu.The transmutation and decay chain is shown below.