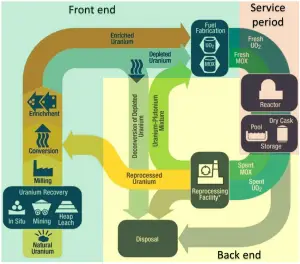
Uranium is often found with copper, phosphates, and other minerals; thus, it is often a co-product of other mining operations. The worldwide production of uranium in 2015 amounted to 60496 tonnes, and Kazakhstan, Canada, and Australia are the top three producers and account for 70% of world uranium production.
Uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium also occurs in seawater and can be recovered from ocean water. But only a few of the uranium ores known contain sufficient uranium (greater than 0.1%) to extract commercially. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.

Natural uranium refers to uranium with the same isotopic ratio found in nature. It consists primarily of isotope 238U (99.28%). Therefore the atomic mass of the uranium element is close to the atomic mass of the 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). The abundance of isotopes in nature is caused by differences in the half-lives. All three naturally-occurring isotopes of uranium (238U, 235U, and 234U) are unstable. On the other hand, these isotopes (except 234U) belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.5×109years for 238U).
Consumption of a 3000MWth (~1000MWe) pressurized water reactor (12-month fuel cycle)
It is an illustrative example, and the following data do not correspond to any reactor design.
- A typical reactor may contain about 100 tonnes of enriched uranium (i.e., about 113 tonnes of uranium dioxide).
- This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods.
- A common fuel assembly contains energy for approximately 4 years of operation at full power.
- Therefore about one-quarter of the core is yearly removed from the spent fuel pool (i.e., about 40 fuel assemblies). At the same time, the remainder is rearranged to a location in the core better suited to its remaining level of enrichment (see Power Distribution).
- This reactor’s annual natural uranium consumption is about 250 tonnes of natural uranium (to produce about 25 tonnes of enriched uranium).
See also: Fuel Consumption
Methods and Techniques of Uranium Mining
As with other types of hard rock mining, there are several methods of extraction. In the case of uranium mining, it strongly depends on the concentration of uranium in the ore. For example, the ore extracted from the Australian Olympic Dam Mine has a concentration of 0.05 %, and most reserves have uranium with a concentration of between 0.1 bis 0.2 %. In Canadian Saskatchewan, the ore mined contains more than 20 % uranium. There are three main methods of extracting uranium:
- Open pit mining. In open pit mining, overburden is removed by drilling and blasting to expose the ore body, which is then mined by blasting and excavation using loaders and dump trucks. Open pit mines require large holes on the surface, larger than the size of the ore deposit, since the pit’s walls must be sloped to prevent collapse. In 2012, the percentage of the mined uranium produced by open pits was 19.9 percent. For example, the Rössing Uranium Mine in Namibia is the longest-running and one of the world’s largest open pit uranium mines.
- Underground mining. Underground uranium mining is, in principle, no different from hard rock mining, and other ores are often mined in association (e.g., copper, gold, silver). The uranium ore is extracted through mechanical means such as blasting, drilling, pneumatic drilling, picks, and shovels and then transported to the surface. Underground mining is usually used for open pit mining when the uranium is too far below the surface. This type of mining exposes underground workers to the highest levels of radon gas. In 2012, the percentage of the mined uranium produced by underground mining was 26.2 percent. For example, the Olympic Dam mine is a large poly-metallic underground mine located in South Australia, 550 km NNW of Adelaide. It is the fourth largest copper deposit and the largest known single deposit of uranium worldwide, although copper is the largest contributor to total revenue.
-
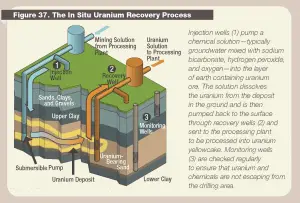
Figure: Source: US Nuclear Regulatory Commission. License: Public Domain In-Situ-Leaching (ISL). In-situ leaching, also known as in-situ recovery, is a mining process different from the conventional method. It uses a chemical process to separate the uranium in the Earth’s crust from the surrounding rock. In-situ is a Latin phrase that translates to “on-site” or “in position.” In-situ leach for uranium has expanded rapidly since the 1990s and is now the predominant method for mining uranium. In this technology, uranium is leached from the in-place ore through an array of regularly spaced wells and is then recovered from the leach solution at a surface plant. The chemical solution is injected into a drilled hole into the rock at the periphery of the uranium deposit. Solutions to dissolve uranium ore are either acid (sulfuric acid or, less commonly, nitric acid) or carbonate (sodium bicarbonate). This liquid loosens the uranium from the rock and binds it; in other words, the uranium is “flushed “out of the rock. Now supplemented with uranium, this solution is brought up to the surface through another borehole. The extracted uranium solution is then filtered through resin beads. The resin beads attract uranium from the solution through an ion exchange process. Uranium-loaded resins are then transported to a processing plant, where U3O8 is separated from the resin beads, and a yellowcake is produced. The ISL method can be used without enormous mining quantities of rock. There are no tailings or waste rock generated. It also impacts the environment and health less than the conventional method, and it is cheaper. On the other hand, there are potential hazards of groundwater contamination. Not all of the contaminated liquid is pumped out. The rock reacts to the chemical solution unpredictably. Therefore, the ISL method can only be performed under certain subsurface conditions. In 2012, the percentage of the mined uranium produced by in-situ leach was 44.9 percent. In this technology, uranium is leached from the in-place ore through an array of regularly spaced wells and is then recovered from the leach solution at a surface plant. For example, the Honeymoon Mine was Australia’s second operating in-situ recovery uranium mine, beginning production in 2011.
Another method of uranium extraction is heap leaching. Heap leaching is an extraction process from ore that has been mined and placed in piles on the surface. Heap leaching is based on using a series of chemical reactions that absorb specific minerals and re-separate them after their division from other earth materials. In 2012, the percentage of the mined uranium produced by heap leaching was 1.7 percent. The remaining 7.3% was derived from mining for other minerals and miscellaneous recovery.