The pascal (Pa) as a unit of pressure measurement is widely used worldwide. It has largely replaced the pounds per square inch (psi) unit, except in some countries that still use the Imperial measurement system, including the United States. For most engineering problems, pascal (Pa) is a fairly small unit, so it is convenient to work with multiples of the pascal: the kPa, the MPa, or the bar. The following list summarizes a few examples:
- Typically most nuclear power plants operate multi-stage condensing steam turbines. These turbines exhaust steam at a pressure well below atmospheric (e.g.,, at 0.08 bar or 8 kPa or 1.16 psia) and in a partially condensed state. In relative units, it is a negative gauge pressure of about – 0.92 bar, – 92 kPa, or – 13.54 psig.
- The Standard Atmospheric Pressure approximates to the average pressure at sea-level at the latitude 45° N. The Standard Atmospheric Pressure is defined at sea-level at 273oK (0oC) and is:
- 101325 Pa
- 1.01325 bar
- 14.696 psi
- 760 mmHg
- 760 torr
- Car tire overpressure is about 2.5 bar, 0.25 MPa, or 36 psig.
- Steam locomotive fire tube boiler: 150–250 psig
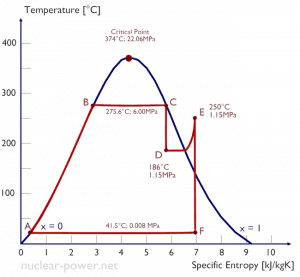
- A high-pressure stage of condensing steam turbine at nuclear power plant operates at steady state with inlet conditions of 6 MPa (60 bar, or 870 psig) t = 275.6°C, x = 1
- A boiling water reactor is cooled and moderated by water like a PWR, but at a lower pressure (e.g.,, 7MPa, 70 bar, or 1015 psig), which allows the water to boil inside the pressure vessel producing the steam that runs the turbines.
- Pressurized water reactors are cooled and moderated by high-pressure liquid water (e.g.,, 16MPa, 160 bar, or 2320 psig). At this pressure, water boils at approximately 350°C (662°F), which provides a subcooling margin of about 25°C.
- The supercritical water reactor (SCWR) is operated at supercritical pressure. The term supercritical in this context refers to the thermodynamic critical point of water (TCR = 374 °C; pCR = 22.1 MPa)
- Common rail direct fuel injection: It features a high-pressure (over 1 000 bar or 100 MPa or 14500 psi) fuel rail on diesel engines.