One of the most familiar forces is the weight of a body, which is the gravitational force that the Earth exerts on the body. In general, gravitation is a natural phenomenon by which all things with mass are brought toward one another. The terms mass and weight are often confused with one another, but it is important to distinguish between them. It is absolutely essential to understand clearly the distinctions between these two physical quantities.
The mass of a certain body will remain constant even if the gravitational acceleration acting upon that body changes. For example, on Earth, an object has a certain mass and a certain weight. When the same object is placed in outer space, away from the Earth’s gravitational field, its mass remains the same, but it is now in a “weightless” condition. This means, in this condition, it will weigh zero because of gravitational acceleration, and, thus, the force will equal zero.
Mass and weight are related: Bodies having a large mass also have a large weight. A large stone is hard to throw because of its large mass and hard to lift off the ground because of its large weight. To understand the relationship between mass and weight, consider a freely falling stone with an acceleration of magnitude g (g = 9.81 m/s2 is the acceleration due to Earth’s gravitational field). Newton’s second law tells us that a force must act to produce this acceleration. If a 1 kilogram stone falls with an acceleration of the required force has magnitude:
F = ma = 1 [kg] x 9.81 [m/s2] = 9.8 [kg m/s2] = 9.8 N
The force that makes the body accelerate downward is its weight. Any body near the surface of the Earth with a mass of 1 kg must weigh 9.8 N to give it the acceleration we observe when it is in free fall.
Example: The weight of a stone on the Earth, on Mars, and the Moon
Weight of a stone on the Earth
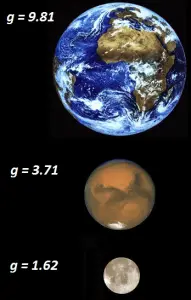 The acceleration due to Earth’s gravitational field is gEarth = 9.81 m/s2.The weight of a stone with mass 1 kg on the Earth can be calculated as:
The acceleration due to Earth’s gravitational field is gEarth = 9.81 m/s2.The weight of a stone with mass 1 kg on the Earth can be calculated as:
FEarth = 1 [kg] x 9.81 [m/s2] = 9.8 [kg m/s2] = 9.8 N
Weight of a stone on the Mars
The acceleration of gravity on Mars is approximately 38% of the acceleration of gravity on the Earth. The acceleration due to Moon’s gravitational field is gMars = 3.71 m/s2.
Therefore the weight of the same stone with mass 1 kg on the Mars is:
FMoon = 1 [kg] x 3.71 [m/s2] = 3.71 [kg m/s2] = 3.71 N
Weight of a stone on the Moon
The acceleration of gravity on the Moon is approximately 1/6 of the acceleration of gravity on the Earth. The acceleration due to Moon’s gravitational field is gMoon = 1.62 m/s2.
Therefore the weight of the same stone with mass 1 kg on the Moon is:
FMoon = 1 [kg] x 1.62 [m/s2] = 1.62 [kg m/s2] = 1.62 N
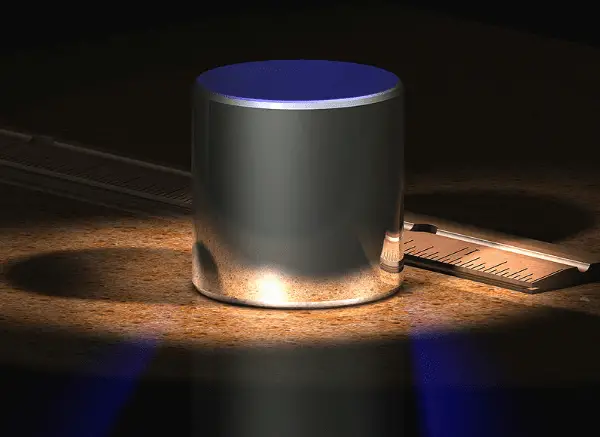
The Standard Kilogram
The usual symbol for mass is m, and its SI unit is the kilogram. The SI standard of mass is a cylinder of platinum and iridium kept at the International Bureau of Weights and Measures near Paris and assigned, by international agreement, a mass of 1 kilogram.