Article Summary & FAQs
What is thermodynamics?
Thermodynamics is the science that deals with energy production, storage, transfer, and conversion. It studies the effects of work, heat, and energy on a system. Despite the fact it is a very broad subject that affects most fields of science, including biology and microelectronics, we will concern mostly with large-scale observations.
Key Facts
- In physics and everyday life, a temperature is an objective comparative measurement of hot or cold based on our sense of touch. This definition is not a simple matter. The kinetic theory of gases provides a microscopic explanation of temperature. It is based on the fact that part of the energy will transfer to the molecule of lower kinetic energy during an elastic collision between a molecule with high kinetic energy and one with low kinetic energy.
- Energy is generally defined as the potential to do work or produce heat. Sometimes it is like the “currency” for performing work. You must have the energy to accomplish work. To do 1 kilojoule of work, you must expend 1 kilojoule of energy.
- The enthalpy is the sum of the internal energy E plus the product of the pressure p and volume V.
- There are four laws of thermodynamics that define fundamental physical quantities (temperature, energy, and entropy) and characterize thermodynamic systems at thermal equilibrium.
- The ideal gas model is used to predict the behavior of gases and is one of the most useful and commonly used substance models ever developed.
- A thermodynamic process is defined as a change from one equilibrium macrostate to another macrostate. The initial and final states are the defining elements of the process.
- The typical thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work while varying pressure, temperature, and other state variables, eventually returning a system to its initial state.
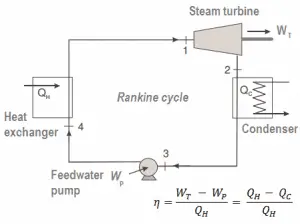
- Today, the Rankine cycle is the fundamental operating cycle of all thermal power plants where an operating fluid is continuously evaporated and condensed.
Thermodynamics
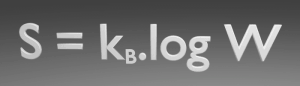 Knowledge of thermodynamics is essential to nuclear engineers who deal with nuclear power reactors. A nuclear power plant (nuclear power station) looks like a standard thermal power station with one exception. The heat source in the nuclear power plant is a nuclear reactor. As is typical in many conventional thermal power stations, the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity.
Knowledge of thermodynamics is essential to nuclear engineers who deal with nuclear power reactors. A nuclear power plant (nuclear power station) looks like a standard thermal power station with one exception. The heat source in the nuclear power plant is a nuclear reactor. As is typical in many conventional thermal power stations, the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity.
Thermodynamics is the science that deals with energy production, storage, transfer, and conversion. It studies the effects of work, heat, and energy on a system. Despite the fact it is a very broad subject that affects most fields of science, including biology and microelectronics, we will concern mostly with large-scale observations. Small-scale interactions will be described in the kinetic theory of gases.
Historically, thermodynamics was born in the 19th century as scientists were first discovering how to build and operate steam engines, particularly through French physicist Nicolas Léonard Sadi Carnot, who introduced the concept of the heat-engine cycle and the principle of reversibility in 1824. Scottish physicist Lord Kelvin was the first to formulate a concise definition of thermodynamics in 1854. Carnot’s work concerned the limitations on the maximum amount of work obtained from a steam engine operating with a high-temperature heat transfer as its driving force. In later years the laws of thermodynamics were developed. Thermodynamics is principally based on four laws that are universally valid when applied to systems that fall within the constraints implied by each.
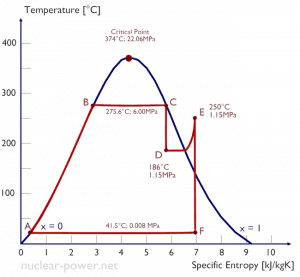
Thermodynamics is both a branch of physics and engineering science. The physicist is normally interested in gaining a fundamental understanding of the physical and chemical behavior of fixed quantities of matter at rest and uses the laws of thermodynamics to relate the properties of matter. Engineers are generally interested in studying energy systems and how they interact with their surroundings. To facilitate this, engineers extend the subject of thermodynamics to the study of open systems, in which heat, work, and mass can be directed into or out of the control volume.
Our goal here will be to introduce thermodynamics as the energy conversion science and introduce some of the fundamental concepts and definitions used in the study of engineering thermodynamics. These fundamental concepts and definitions will be further applied to energy systems and finally to thermal or nuclear power plants.
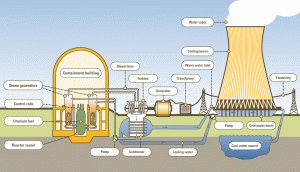
A typical nuclear power plant has an electric-generating capacity of 1000 MWe. The heat source in the nuclear power plant is a nuclear reactor. As is typical in all conventional thermal power stations, the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity. The turbines are heat engines subject to the efficiency limitations imposed by the second law of thermodynamics. In modern nuclear power plants, the overall thermodynamic efficiency is about one-third (33%), so 3000 MWth of thermal power from the fission reaction is needed to generate 1000 MWe of electrical power.
In the following sections, we will deal with the problem, how to transform the thermal energy generated inside the reactor into electrical energy in a most effective way.
Laws of Thermodynamics
There are four laws of thermodynamics that define fundamental physical quantities (temperature, energy, and entropy) and characterize thermodynamic systems at thermal equilibrium. These are considered as one of the most important laws in all of physics. The laws are as follows:
If two systems are both in thermal equilibrium with a third, they are in thermal equilibrium.
This law provides a definition and method of defining temperatures, perhaps the most important intensive property of a system when dealing with thermal energy conversion problems.
The first law of thermodynamics:
The increase in internal energy of a closed system is equal to the heat supplied to the system minus its work.
This law is the principle of conservation of energy. It is the most important law for analyzing most systems and quantifying how thermal energy is transformed to other forms of energy. It follows perpetual motion machines of the first kind are impossible.
The second law of thermodynamics:
The entropy of any isolated system never decreases. In a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems increases.
This law indicates the irreversibility of natural processes. Reversible processes are a useful and convenient theoretical fiction but do not occur in nature. From this law, it is impossible to construct a device that operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body. It follows perpetual motion machines of the second kind are impossible.
The entropy of a system approaches a constant value as the temperature approaches absolute zero.
Based on empirical evidence, this law states that the entropy of a pure crystalline substance is zero at the absolute zero of temperature, 0 K and that it is impossible using any process, no matter how idealized, to reduce the temperature of a system to absolute zero in a finite number of steps. This allows us to define a zero point for the thermal energy of a body.
Popular Version of the Laws of Thermodynamics
0. You must play the game.
1. You can’t win; you can only break even.
2. You can only break even at absolute zero.
3. You can’t reach absolute zero.
Extensive Properties – Intensive Properties
What is Energy
Source: hyperphysics.phy-astr.gsu.edu
The term energy is very broad, and it has many definitions. Technically, energy is a scalar physical quantity that is associated with the state of one or more objects. Energy is generally defined as the potential to do work or produce heat. Sometimes it is like the “currency” for performing work. You must have the energy to accomplish work. To do 1 kilojoule of work, you must expend 1 kilojoule of energy. It must be added. This interpretation can be misleading because energy is not necessarily available to do work.
One of the most wonderful properties of the universe is that energy can be transformed from one type to another and transferred from one object to another. Moreover, when transformed from one type to another and transferred from one object to another, the total amount of energy is always the same. It is one of the elementary properties of the universe.
For example, burning gasoline to power cars is an energy conversion process we rely on. The chemical energy in gasoline is converted to thermal energy, then converted to mechanical energy that makes the car move. The mechanical energy has been converted to kinetic energy. When we use the brakes to stop a car, that kinetic energy is converted by friction back to heat or thermal energy.
Heat Engines
Energy sources have always played a very important role in the development of human society. Energy is generally defined as the potential to do work or produce heat. Sometimes it is like the “currency” for performing work. One of the most wonderful properties of the universe is that energy can be transformed from one type to another and transferred from one object to another.
In general, it is easy to produce thermal energy by doing work, for example, by any frictional process. But to get work from thermal energy is more difficult. It is closely associated with the concept of entropy. For example, electricity is particularly useful since it has very low entropy (is highly ordered) and can be converted into other forms of energy very efficiently.
Sometimes, mechanical energy is directly available, for example, wind power and hydropower. But most of our energy comes from burning fossil fuels (coal, oil, and gas) and nuclear reactions. At present, fossil fuel is still the world’s predominant energy source. But the burning of fossil fuels generates only thermal energy. Therefore these energy sources are so-called “primary energy sources” that must be converted to the secondary energy source, so-called energy carriers (electrical energy, etc.). A heat engine must be used to convert thermal energy into another form of energy.
In general, a heat engine is a device that converts chemical energy to heat or thermal energy and then to mechanical energy or electrical energy.
The Rankine cycle closely describes the processes in steam-operated heat engines commonly found in most thermal power plants.
Many heat engines operate cyclically, adding energy in the form of heat in one part of the cycle and using that energy to do useful work in another part of the cycle.
For example, as is typical in all conventional thermal power plants, the heat is used to generate steam which drives a steam turbine connected to a generator that produces electricity. Steam generators, steam turbines, condensers, and feedwater pumps constitute a heat engine subject to the efficiency limitations imposed by the second law of thermodynamics. In modern nuclear power plants, the overall thermodynamic efficiency is about one-third (33%), so 3000 MWth of thermal power from the fission reaction is needed to generate 1000 MWe of electrical power.