Terrestrial radiation refers to radiation sources in the soil, water, vegetation, and the Earth’s mantle. The major isotopes of concern for terrestrial radiation are potassium, uranium, and the decay products of uranium, such as thorium, radium, and radon. When the Earth was formed, many radioactive elements were formed. After four billion years, all the shorter-lived isotopes have decayed. But some of these isotopes have very long half-lives, billions of years, and are still present. These radionuclides are known as primordial radionuclides and contribute to an individual’s annual dose. Because most natural radioactive isotopes are heavy, more than one disintegration is necessary before reaching a stable atom. This sequence of unstable atomic nuclei and their modes of decays, which leads to a stable nucleus, is known as the radioactive series. Radioactive decays of members of these series generate heat.
Liquid Earth’s Core
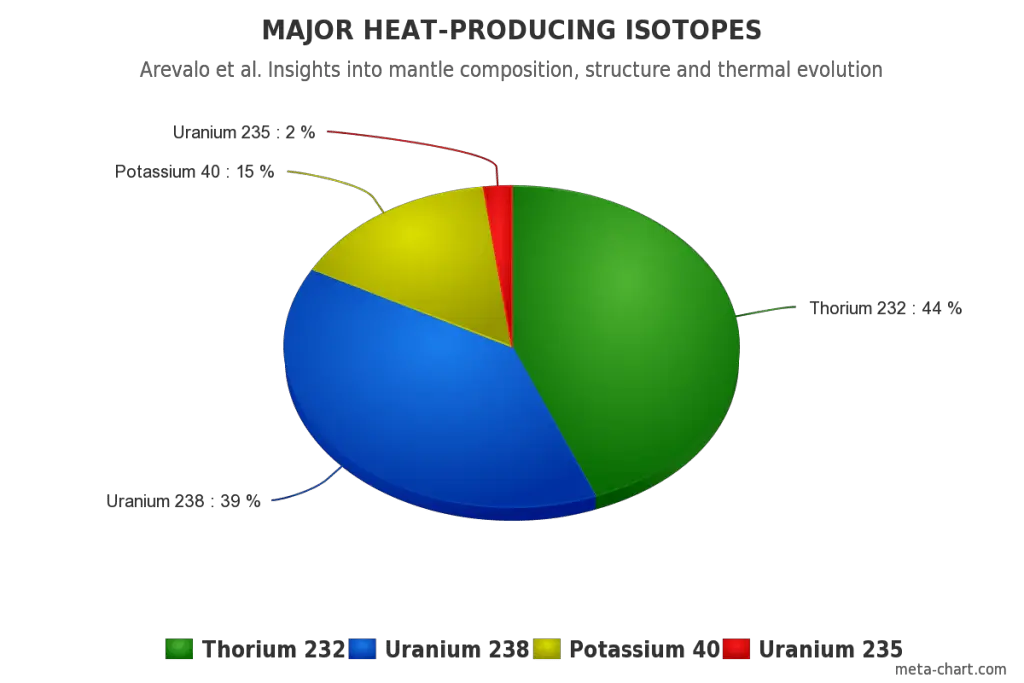 All three naturally-occurring isotopes of uranium (238U, 235U, and 234U) and naturally-occurring isotope of thorium have a very long half-life (e.g., 4.47×109 years for 238U). Because of this long half-life, uranium and thorium are weakly radioactive and contribute to low levels of natural background radiation in the environment. These isotopes are alpha radioactive (emitting alpha particles), but they can also rarely undergo spontaneous fission.
All three naturally-occurring isotopes of uranium (238U, 235U, and 234U) and naturally-occurring isotope of thorium have a very long half-life (e.g., 4.47×109 years for 238U). Because of this long half-life, uranium and thorium are weakly radioactive and contribute to low levels of natural background radiation in the environment. These isotopes are alpha radioactive (emitting alpha particles), but they can also rarely undergo spontaneous fission.
All naturally-occurring isotopes belong to primordial nuclides because their half-life is comparable to the age of the Earth (~4.54×109 years). Uranium has the second-highest atomic mass of these primordial nuclides, lighter only than plutonium. Moreover, the decay heat of uranium and thorium and their decay products (e.g., radon, radium, etc.) contribute to heating the Earth’s core. Together with potassium-40 in the Earth’s mantle, these elements are the main source of heat that keeps the Earth’s core liquid.