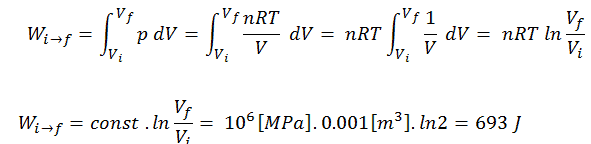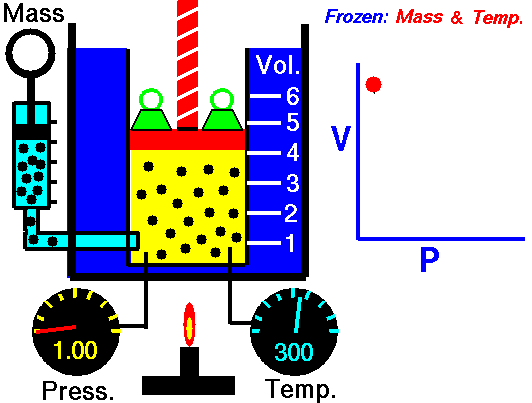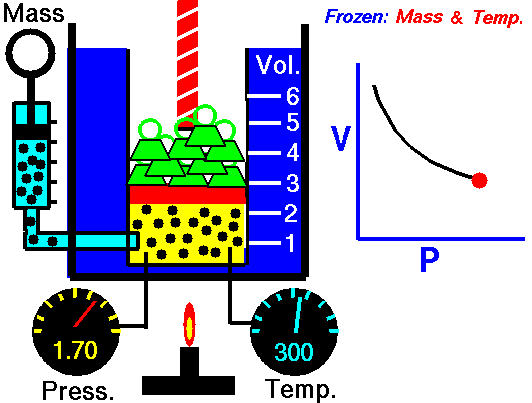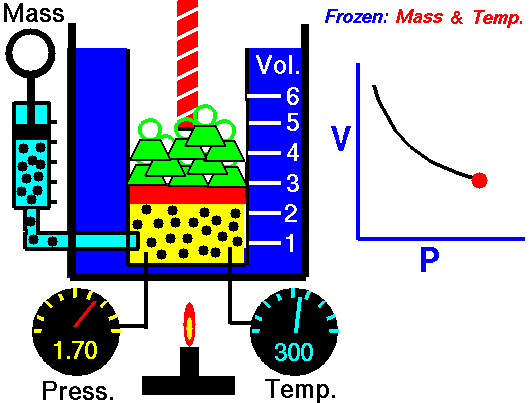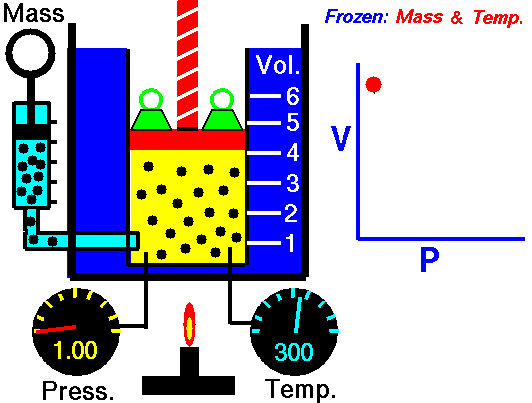
Assume an isothermal expansion of helium (i → f) in a frictionless piston (closed system). The gas expansion is propelled by the absorption of heat energy Qadd. The gas expands from an initial volume of 0.001 m3, and simultaneously the external load of the piston slowly and continuously decreases from 1 MPa to 0.5 MPa. Since helium behaves almost as an ideal gas, use the ideal gas law to calculate the final volume of the chamber and then calculate the work done by the system when the temperature of the gas is equal to 400 K.
Solution:
The final volume of the gas, Vf, can be calculated using p, V, T Relation for isothermal process:
piVi = pfVf ⇒ Vf = piVi / pf = 2 x 0.001 m3 = 0.002 m3
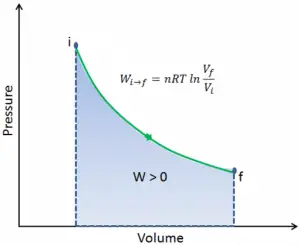
To calculate such processes, we would need to know how pressure varies with volume for the actual process by which the system changes from state i to state f. Since during this process the internal pressure was not constant, the p∆V work done by the piston must be calculated using the following integral:
By convention, a positive value for work indicates that the system in its surroundings does work. A negative value indicates that work is done on the system by its surroundings. The pΔV work is equal to the area under the process curve plotted on the pressure-volume diagram.