Terrestrial radiation refers to radiation sources in the soil, water, and vegetation. The major isotopes of concern for terrestrial radiation are potassium, uranium, and the decay products of uranium, such as thorium, radium, and radon. Note that terrestrial radiation includes an external exposure caused by these radionuclides. An internal dose caused by these radionuclides is discussed in: Internal Source of Radiation.
These radionuclides are in trace amounts all around us. When the Earth was formed, many radioactive elements were formed. After four billion years, all the shorter-lived isotopes have decayed. But some of these isotopes have very long half-lives, billions of years, and are still present. These radionuclides are known as primordial radionuclides and contribute to the annual dose of an individual. Because most natural radioactive isotopes are heavy, more than one disintegration is necessary before reaching a stable atom. This sequence of unstable atomic nuclei and their modes of decays, which leads to a stable nucleus, is known as the radioactive series.
Dose from Terrestrial Radiation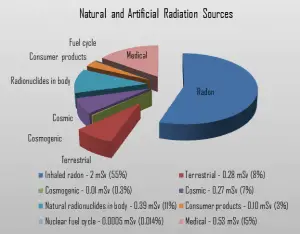
Low levels of uranium, thorium and their decay products are found everywhere. Some of these materials are ingested with food and water, while others, such as radon, are inhaled. The dose from terrestrial sources also varies in different parts of the world, and locations with higher concentrations of uranium and thorium in their soil have higher dose levels. The average dose rate that originates from terrestrial nuclides (except radon exposure) is about 0.057 µGy/hr. The maximum values have been measured on monazite sand in Guarapari, Brazil (up to 50 µGy/hr and in Kerala, India (about 2 µGy/hr), and on rocks with a high radium concentration in Ramsar, Iran (from 1 to 10 µGy/hr).
The major isotopes of concern for terrestrial radiation are uranium and the decay products of uranium, such as thorium, radium, and radon. Radon is usually the largest natural source of radiation contributing to the exposure of public members, sometimes accounting for half the total exposure from all sources. It is so important that it is usually treated separately. The average annual radiation dose to a person from radon and its decay products is about 2 mSv/year, and it may vary over many orders of magnitude from place to place.
Radiation from Thorium and its Decay Products
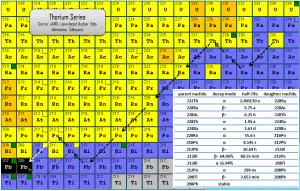 Thorium cascade significantly influences radioactivity(disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural thorium-containing sample, whether metal, compound or mineral. For example, pure thorium-232 is weakly radioactive (proportional to its long half-life). Still, a thorium ore is about 10 times more radioactive than the pure thorium-232 metal because of its daughter isotopes (e.g., radon, radium, etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain, they also generate radon, a heavy, inert, naturally occurring radioactive gas.
Thorium cascade significantly influences radioactivity(disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural thorium-containing sample, whether metal, compound or mineral. For example, pure thorium-232 is weakly radioactive (proportional to its long half-life). Still, a thorium ore is about 10 times more radioactive than the pure thorium-232 metal because of its daughter isotopes (e.g., radon, radium, etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain, they also generate radon, a heavy, inert, naturally occurring radioactive gas.