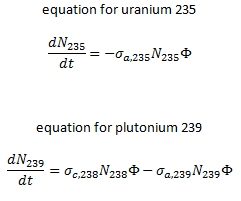
The exact evolution of isotopic changes is usually modeled mathematically by a set of differential equations known as evolution equations. These equations describe the rate of burnup of U-235, the rate of buildup of Pu-239, production of Pu-240 and Pu-241, the buildup of neutron-absorbing fission products and the overall rate of reactivity change in the reactor due to the changing composition of the fuel. The evolution equation can be constructed for each isotope. For example:
Special reference: W. M. Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-1.
Special reference: Paul Reuss, Neutron Physics, EDP Sciences, 2008, ISBN: 2759800415.
Isotopic Changes – Summary
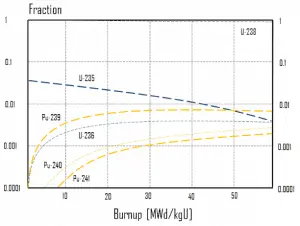
In summary, it can be seen for fuel burnup of 40 GWd/tU:
- Approximately 3 – 4% of the heavy nuclei are fissioned.
- About two-thirds of these fissions come directly from uranium 235, and the other third comes from plutonium produced from uranium 238. The contribution significantly increases as the fuel burnup increases.
- The removed fuel (spent nuclear fuel) still contains about 96% reusable material. It must be removed due to decreasing kinf of an assembly or, in other words, it must be removed due to accumulation of fission products with significant absorption cross-section.
- Discharged fuel contains abour 0.8% of plutonium and about 1% of uranium 235. It must be noted, there is a significant content (about 0.5%) of uranium 236, which is neither a fissile isotope, nor a fertile isotope.