Photoelectric Effect
- The photoelectric effect dominates at low-energies of gamma rays.
- The photoelectric effect leads to the emission of photoelectrons from matter when light (photons) shines upon them.
- The maximum energy an electron can receive in any one interaction is hν.
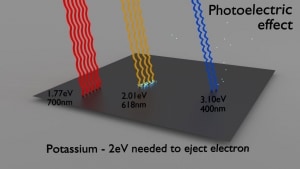
- The photoelectric effect only emits electrons if the photon reaches or exceeds threshold energy.
- A free-electron (e.g.,, from an atomic cloud) cannot absorb the entire energy of the incident photon. This is a result of the need to conserve both momentum and energy.
- The cross-section for the emission of n=1 (K-shell) photoelectrons is higher than that of n=2 (L-shell) photoelectrons. This is a result of the need to conserve momentum and energy.
Definition of Photoelectric effect
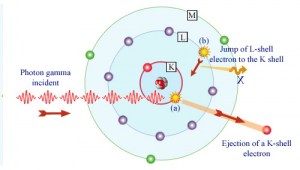
In the photoelectric effect, a photon undergoes an interaction with an electron that is bound in an atom. The incident photon completely disappears in this interaction, and the atom ejects an energetic photoelectron from one of its bound shells. The kinetic energy of the ejected photoelectron (Ee) is equal to the incident photon energy (hν) minus the binding energy of the photoelectron in its original shell (Eb).
Ee=hν-Eb
Therefore, photoelectrons are only emitted by the photoelectric effect if the photon reaches or exceeds threshold energy – the electron’s binding energy – the work function of the material. For gamma rays with energies of more than hundreds keV, the photoelectron carries off the majority of the incident photon energy – hν. Following a photoelectric interaction, an ionized absorber atom is created with a vacancy in one of its bound shells. An electron from a shell will quickly fill this vacancy with lower binding energy (other shells) or capture a free electron from the material. The rearrangement of electrons from other shells creates another vacancy, which, in turn, is filled by an electron from an even lower binding energy shell. Therefore a cascade of more characteristic X-rays can also be generated. The probability of characteristic x-ray emission decreases as the atomic number of the absorber decreases. Sometimes, the emission of an Auger electron occurs.