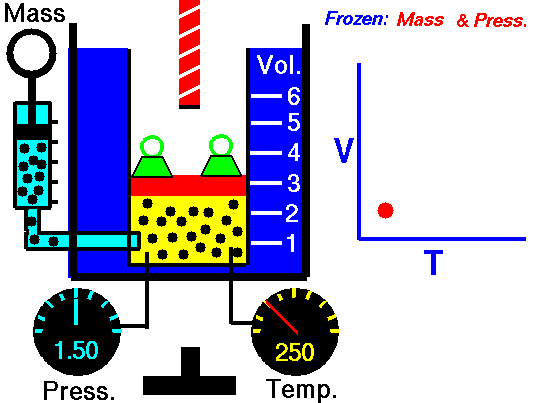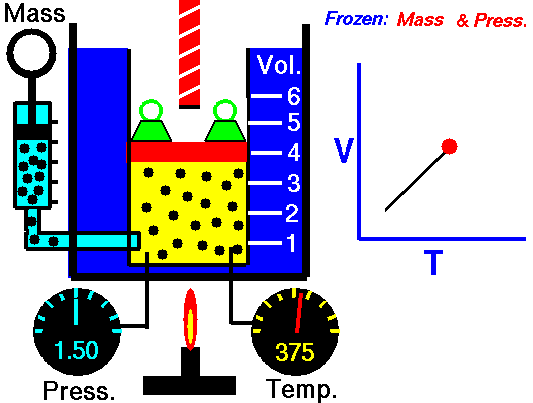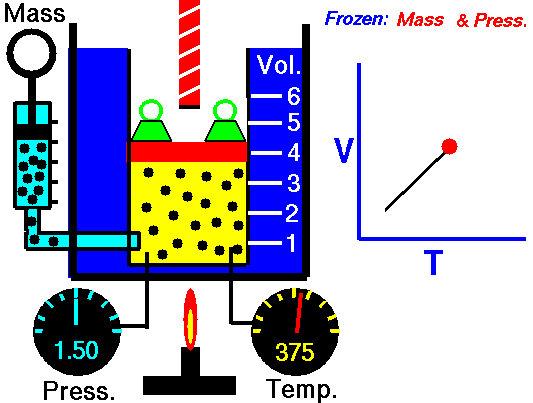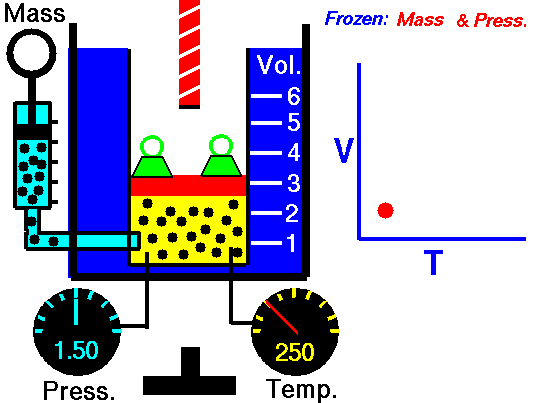
For a fixed mass of gas at constant pressure, the volume is directly proportional to the Kelvin temperature.
Charles’s Law is one of the gas laws. At the end of the 18th century, a French inventor and scientist, Jacques Alexandre César Charles, studied the relationship between the volume and the temperature of a gas at constant pressure. The results of certain experiments with gases at relatively low pressure led Jacques Alexandre César Charles to formulate a well-known law. It states that:
For a fixed mass of gas at constant pressure, the volume is directly proportional to the Kelvin temperature.
That means that, for example, if you double the temperature, you will double the volume. If you halve the temperature, you will halve the volume.
You can express this mathematically as:
V = constant . T
Yes, it seems to be identical to the isobaric process of an ideal gas. These results are fully consistent with the ideal gas law, which determinates that the constant is equal to nR/p. If you rearrange the pV = nRT equation by dividing both sides by p, you will obtain:
V = nR/p . T
where nR/p is constant and:
- p is the absolute pressure of the gas
- n is the amount of substance
- T is the absolute temperature
- V is the volume
- R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
In this equation, the symbol R is the universal gas constant that has the same value for all gases—namely, R = 8.31 J/mol K.