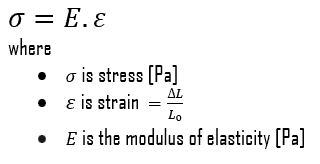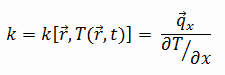High purity aluminium is a soft material with the ultimate strength of approximately 10 MPa, which limits its usability in industrial applications. Aluminium of commercial purity (99-99.6%) becomes harder and stronger due to the presence of impurities, especially Si and Fe. But when alloyed, aluminium alloys are heat treatable, which significantly changes their mechanical properties.
 Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, and Zn. Aluminium alloy compositions are registered with The Aluminum Association. The aluminium alloys are divided into 9 families (Al1xxx to Al9xxx). The different families of alloys and the major alloying elements are:
Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, and Zn. Aluminium alloy compositions are registered with The Aluminum Association. The aluminium alloys are divided into 9 families (Al1xxx to Al9xxx). The different families of alloys and the major alloying elements are:
- 1xxx: no alloying elements
- 2xxx: Copper
- 3xxx: Manganese
- 4xxx: Silicon
- 5xxx: Magnesium
- 6xxx: Magnesium and silicon
- 7xxx: Zinc, magnesium, and copper
- 8xxx: other elements which are not covered by other series
There are also two principal classifications, namely casting alloys and wrought alloys, which are further subdivided into the categories of heat-treatable and non-heat-treatable. Aluminium alloys containing alloying elements with limited solid solubility at room temperature and with the strong temperature dependence of solid solubility (for example, Cu) can be strengthened by a suitable thermal treatment (precipitation hardening). The strength of heat-treated commercial Al alloys exceeds 550 MPa.
The mechanical properties of aluminium alloys highly depend on their phase composition and microstructure. Among others, high strength can be achieved by introducing a high volume fraction of fine, homogeneously distributed second phase particles and refining the grain size. In general, aluminium alloys are characterized by a relatively low density (2.7 g/cm3 compared to 7.9 g/cm3 for steel), high electrical and thermal conductivities, and resistance to corrosion in some common environments, including the ambient atmosphere. The chief limitation of aluminum is its low melting temperature (660°C), which restricts the maximum temperature at which it can be used. For general production, the 5000 and 6000 series alloys provide adequate strength, good corrosion resistance, high toughness, and ease of welding.
 Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical, and electronic applications. It has been the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial and civil aircraft airframes are made from aluminium alloys; civil aviation would not be economically viable without aluminium. The automotive industry now includes aluminium as engine castings, wheels, radiators, and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast and made of aluminium alloys.
Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical, and electronic applications. It has been the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial and civil aircraft airframes are made from aluminium alloys; civil aviation would not be economically viable without aluminium. The automotive industry now includes aluminium as engine castings, wheels, radiators, and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast and made of aluminium alloys.
Strengthening Mechanisms of Aluminium Alloys
The strength of aluminum alloys can be modified through various combinations of cold working, alloying, and heat treating. For example, a microstructure with finer grains typically results in higher strength and superior toughness than the same alloy with physically larger grains. In the case of grain size, there may also be a tradeoff between strength and creep characteristics. Other strengthening mechanisms are achieved at the expense of lower ductility and toughness.
- Solid Solution Hardening (alloying). Atoms of different elements dissolved in the matrix phase can lead to its strengthening by solid solution strengthening. The solute may incorporate into the solvent crystal lattice substitutionally by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. This imposes lattice strains on surrounding atoms resulting in a lattice strain field. Even small amounts of solute can affect the electrical and physical properties of the solvent. Manganese and magnesium are examples of elements added to aluminum to strengthen. Solid solution strengthening occurs in 3xxx and 5xxx alloys by adding manganese (3xxx) and magnesium (5xxx) to aluminum.
- Strain Hardening (cold working). Strain hardening also called work-hardening, or cold-working, is a strengthening method often used in materials whose strength cannot be increased by heat treatment, e.g., by changes in their phase composition. It is called cold-working because the plastic deformation must occur at a temperature low enough that atoms cannot rearrange themselves. It is a process of making a metal harder and stronger through plastic deformation. When a metal is plastically deformed, dislocations move, generating additional dislocations. Dislocations can move if the atoms from one of the surrounding planes break their bonds and rebond with the atoms at the terminating edge. The dislocation density in a metal increases with deformation or cold work because of dislocation multiplication or the formation of new dislocations. The more dislocations within a material, the more they interact and become pinned or tangled. This will result in a decrease in the mobility of the dislocations and a strengthening of the material. Cold working involves the reduction in the thickness of a material. Plate and sheets of different thicknesses are produced by cold rolling. Wire and tubes of different diameters and wall thicknesses are produced by drawing. All aluminum alloys can be strengthened by cold working.
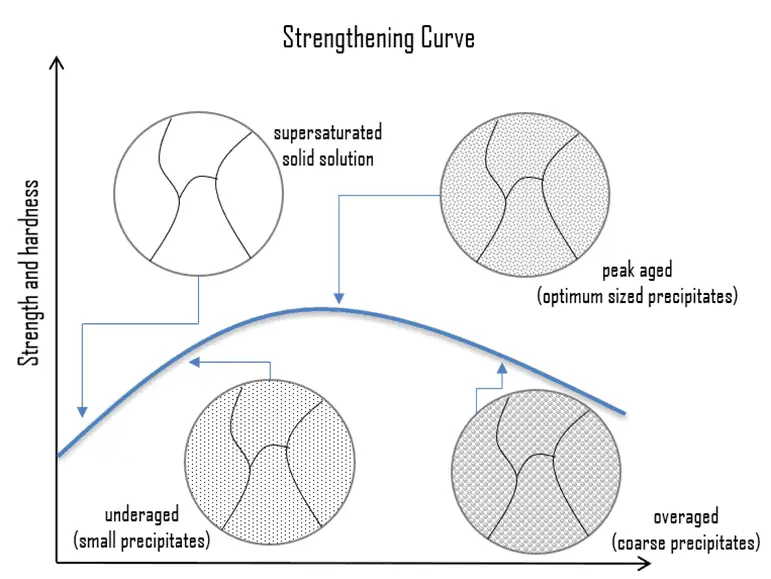 Precipitation (age) Hardening. Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique based on forming extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second-phase particles present further types of obstacles for dislocation movement. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles that hinder the dislocation motion are the strain field around the second phase particles, the second phase particles themselves, or both. Precipitation hardening increases the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, some steels, and stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength. In the case of aluminium alloys, precipitation strengthening can increase the yield strength of aluminium from about five times up to about fifteen times that of unalloyed aluminium. Especially 2xxx series, alloyed with copper, can be precipitation hardened to strengths comparable to steel. In terms of age hardening, solution annealed aluminum-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding it at that temperature for a specified time. This process is called artificial aging.
Precipitation (age) Hardening. Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique based on forming extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second-phase particles present further types of obstacles for dislocation movement. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles that hinder the dislocation motion are the strain field around the second phase particles, the second phase particles themselves, or both. Precipitation hardening increases the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, some steels, and stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength. In the case of aluminium alloys, precipitation strengthening can increase the yield strength of aluminium from about five times up to about fifteen times that of unalloyed aluminium. Especially 2xxx series, alloyed with copper, can be precipitation hardened to strengths comparable to steel. In terms of age hardening, solution annealed aluminum-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding it at that temperature for a specified time. This process is called artificial aging.- Dispersion Hardening. Dispersion hardening involves including small, hard particles in the metal, thus restricting the movement of dislocations and raising the strength properties. It is, in many ways, very similar to age hardening. The difference lies in the precipitates themselves—the particles are chosen because of their thermal stability, that is, their resistance to particle coarsening or growth at high temperatures. Dispersoid particles influence the grain structure, and the increase in strength is due to the grain structure formed due to the presence of dispersoids.
- Grain Refinement (small grain size). The size of the grain determines the properties of the metal. For example, smaller grain size increases tensile strength and tends to increase ductility. Larger grain size is preferred for improved high-temperature creep properties. Decreasing the grain size also is an effective way to increase ductility. When grain size is reduced, there are more grains with a greater number of arbitrarily aligned slip planes for the dislocations in the grains. This provides more opportunity for some slips to occur in a stressed material. Thus, grain refinement provides an important means to improve strength, ductility, and toughness. Many other strengthening mechanisms are achieved at the expense of ductility and toughness.
Reactor use of Aluminium
Aluminium, with its low cost, low thermal neutron absorption (0.24 barns), and freedom from corrosion at low temperature, is ideally suited for research or training reactors (e.g., cladding material) in the low kilowatt power and low temperature operating ranges. Generally, at high temperatures (in water, corrosion limits the use of aluminium to temperatures near 100°C), the relatively low strength and poor corrosion properties of aluminium make it unsuitable as a structural material in power reactors due to hydrogen generation.
Example – Aluminium Alloys – Series 2000 – Duralumin
Aluminium alloys of the 2000 series are alloyed with copper, and they can be precipitation hardened to strengths comparable to steel. Formerly referred to as duralumin, they were once the most common aerospace alloys but were susceptible to stress corrosion cracking and are increasingly replaced by 7000 series in new designs. In addition to aluminium, the main materials in duralumin are copper, manganese, and magnesium.
Duralumin (also called duraluminum, duraluminium, duralum, dural(l)ium, or dural) is a strong, lightweight alloy of aluminium discovered in 1910 by Alfred Wilm a German metallurgist. He discovered that after quenching, an aluminium alloy containing 4% copper would slowly harden when left at room temperature for several days. This process is now known as natural aging. He also designed an alloy (Duralumin) suitable for strengthening by this process in what is now known as precipitation hardening. Although an explanation for the phenomenon was not provided until 1919, duralumin was one of the first “age hardening” alloys used.
In terms of age hardening, solution annealed aluminium-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. At room temperature, the solubility of copper in aluminium drops to a small fraction of 1%. At this point, the copper solute is locked inside the aluminium lattice (matrix) but must “precipitate” out of the supersaturated aluminium lattice. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding it at that temperature for a specified time. This process is called artificial aging.
Duralumin is relatively soft, ductile, and easily workable under normal temperatures. The alloy can be rolled, forged, and extruded into various forms and products. The lightweight and high strength of duralumin, when compared to steel, enabled its application in aircraft construction. Although adding copper improves strength, it also makes these alloys susceptible to corrosion. The electrical and heat conductivity of duralumin is less than that of pure aluminium and more than that of steel.
Example – Aluminium Alloys – 6061 Alloy
 In general, 6000 series aluminium alloys are alloyed with magnesium and silicon. Alloy 6061 is one of the most widely used alloys in the 6000 Series. It has good mechanical properties, and it is easy to machine. It is weldable and can be precipitation hardened, but not to the high strengths that 2000 and 7000 can reach. It has very good corrosion resistance and very good weldability, although reduced strength in the weld zone. The mechanical properties of 6061 depend greatly on the material’s temper or heat treatment. Compared to 2024 alloy, 6061 is more easily worked and remains resistant to corrosion even when the surface is abraded.
In general, 6000 series aluminium alloys are alloyed with magnesium and silicon. Alloy 6061 is one of the most widely used alloys in the 6000 Series. It has good mechanical properties, and it is easy to machine. It is weldable and can be precipitation hardened, but not to the high strengths that 2000 and 7000 can reach. It has very good corrosion resistance and very good weldability, although reduced strength in the weld zone. The mechanical properties of 6061 depend greatly on the material’s temper or heat treatment. Compared to 2024 alloy, 6061 is more easily worked and remains resistant to corrosion even when the surface is abraded.
This standard structural alloy, one of the most versatile heat-treatable alloys, is popular for medium to high strength requirements and has good toughness characteristics. Applications range from aircraft components (aircraft structures, such as wings and fuselages) to automotive parts such as the chassis of the Audi A8. 6061-T6 is widely used for bicycle frames and components.
Properties of Aluminium Alloys
Material properties are intensive properties, which means they are independent of the amount of mass and may vary from place to place within the system at any moment. Materials science involves studying materials’ structure and relating them to their properties (mechanical, electrical, etc.). Once materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and how it has been processed into its final form.
Mechanical Properties of Aluminium Alloys
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial, and engineers must consider them.
Strength of Aluminium Alloys
In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. The strength of materials considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. The strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
The ultimate tensile strength of 2024 aluminium alloy depends greatly on the temper of the material, but it is about 450 MPa.
The ultimate tensile strength of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper, it is about 290 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
Yield Strength
The yield strength of 2024 aluminium alloy depends greatly on the temper of the material, but it is about 300 MPa.
The yield strength of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper, it is about 240 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically. In contrast, the yield point is where nonlinear (elastic + plastic) deformation begins. Before the yield point, the material will deform elastically and return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behavior termed a yield point phenomenon. Yield strengths vary from 35 MPa for low-strength aluminum to greater than 1400 MPa for high-strength steel.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of 2024 aluminium alloy is about 76 GPa.
Young’s modulus of elasticity of 6061 aluminium alloy is about 69 GPa.
Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to limiting stress, a body will be able to recover its dimensions on the removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position, and all the atoms are displaced the same amount and maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions, and no permanent deformation occurs. According to Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
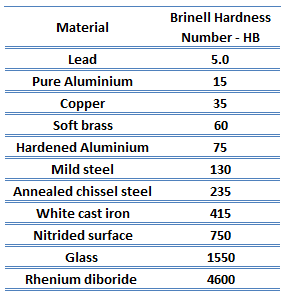
The hardness of Aluminium Alloys
Brinell hardness of 2024 aluminium alloy depends greatly on the temper of the material, but it is approximately 110 MPa.
Brinell hardness of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper, it is approximately 95 MPa.
Rockwell hardness test is one of the most common indentation hardness tests developed for hardness testing. In contrast to the Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position, and the major load is applied, then removed while maintaining the minor load. The difference between the penetration depth before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Aluminium Alloys
Thermal properties of materials refer to the response of materials to changes in their temperature and the application of heat. As a solid absorbs energy in the form of heat, its temperature rises, and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of Aluminium Alloys
The melting point of 2024 aluminium alloy is around 570°C.
The melting point of 6061 aluminium alloy is around 600°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition where the solid and liquid can exist in equilibrium.
Thermal Conductivity of Aluminium Alloys
The thermal conductivity of 2024 aluminium alloy is 140 W/(m. K).
The thermal conductivity of 6061 aluminium alloy is 150 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It measures a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas). Therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature, and for vapors, it also depends upon pressure. In general:
Most materials are nearly homogeneous. Therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz). However, for an isotropic material, the thermal conductivity is independent of the transfer direction, kx = ky = kz = k.