A chemical bond is a lasting attraction between these atoms, ions, or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life.
Hydrogen Bond
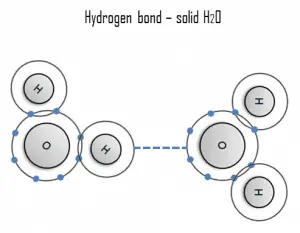 A hydrogen bond can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom covalently bound to a more electronegative atom or group.
A hydrogen bond can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom covalently bound to a more electronegative atom or group.
Hydrogen is a strong example of an interaction between two permanent dipoles. The large difference in electronegativities between hydrogen and any fluorine, nitrogen, and oxygen, coupled with their lone pairs of electrons, causes strong electrostatic forces between molecules. A ubiquitous example of a hydrogen bond is found between water molecules. Hydrogen bonds are responsible for the high boiling points of water. Each H2O molecule has two hydrogen atoms that can bond to oxygen atoms, and in addition, its single O atom can bond to two hydrogen atoms of other H2O molecules. Thus, each water molecule participates in four hydrogen bonds for solid ice, helping to create an open hexagonal lattice. Liquid water’s high boiling point is due to the high number of hydrogen bonds each molecule can form relative to its low molecular mass. Due to the difficulty of breaking these bonds, water has a very high boiling point, melting point, and viscosity compared to similar liquids not conjoined by hydrogen bonds.